Prodine
 | |
| Systematic (IUPAC) name | |
|---|---|
|
(1,3-Dimethyl-4-phenylpiperidin-4-yl) propanoate | |
| Clinical data | |
| Legal status |
|
| Identifiers | |
| CAS Number |
77-20-3 468-59-7 (beta) |
| ATC code | None |
| PubChem | CID 204163 |
| ChemSpider |
176845 |
| UNII |
21J54X4Z4Z |
| ChEMBL |
CHEMBL14309 |
| Chemical data | |
| Formula | C16H23NO2 |
| Molar mass | 261.359 g/mol |
| |
| |
| | |
Prodine (trade names Prisilidine and Nisentil) is an opioid analgesic that is an analog of pethidine (meperidine). It was developed in Germany in the late 1940s.
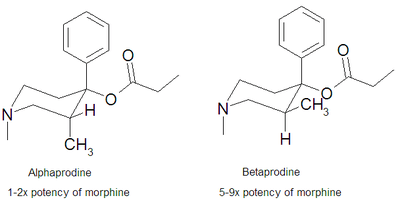
Several 1-alkyl-4-phenyl-4-acyloxypiperidines have been prepared and found to possess significant analgesic action.[1] Those compounds with the 4-propionoxy substituent appear to be the most potent analgesics. Ziering and Lee found that embellishing this structure via the incorporation of a 3-methyl group into the piperidine nucleus greatly enhanced the pharmacological activity.[2]
There are two isomers of prodine, alphaprodine and betaprodine.[3] Betaprodine is some 5x more potent than alphaprodine,[4] but is metabolised more rapidly, and only alphaprodine was developed for medicinal use. It has similar activity to pethidine, but with a faster onset of action and shorter duration.[5]
Alphaprodine has a DEA ACSCN of 9010 and 2013 manufacturing quota of 3 grammes; betaprodine has an ACSCN of 9611 and a 2 gramme quota.
Alphaprodine was sold under several brand names, mainly Nisentil and Prisilidine. It was mainly used for pain relief in childbirth[6] and dentistry,[7] as well as for minor surgical procedures. Alphaprodine has a duration of action of 1 to 2 hours and 40 to 60 mg is equal to 10 mg of morphine via the subcutaneous route.
Prodine has similar effects to other opioids, and produces analgesia, sedation and euphoria. Side effects can include itching, nausea and potentially serious respiratory depression which can be life-threatening. Respiratory depression can be a problem with alphaprodine even at normal therapeutic doses.[8] Unlike pethidine, prodine does not produce toxic metabolites and is therefore more suitable for high dose therapy.
See also
References
- ↑ Ziering, A.; Berger, L. E. O.; Heineman, S. D.; Lee, J. (1947). "Piperidine Derivatives. Part III. 4-Arylpiperidines". The Journal of Organic Chemistry 12 (6): 894. doi:10.1021/jo01170a022. PMID 18919742.
- ↑ Ziering, A.; Lee, J. (1947). "Piperidine Derivatives. V. 1,3-Dialkyl-4-Aryl-4-Acyloxypiperidines". The Journal of Organic Chemistry 12 (6): 911–4. doi:10.1021/jo01170a024. PMID 18919744.
- ↑ Beckett AH, Walker J (1955). "The configuration of alphaprodine and betaprodine". Journal of Pharmacy and Pharmacology 7 (1): 1039–1045. doi:10.1111/j.2042-7158.1955.tb12115.x. PMID 13278850.
- ↑ John Bedford Stenlake. Foundations of Molecular Pharmacology. ISBN 0-485-11171-3.
- ↑ Fung DL, Asling JH, Eisele JH, Martucci R (1980). "A comparison of alphaprodine and meperidine pharmacokinetics". Journal of Clinical Pharmacology 20 (1): 37–41. doi:10.1002/j.1552-4604.1980.tb01664.x. PMID 7358866.
- ↑ Burnett RG, White CA (1966). "Alphaprodine for continuous intravenous obstetric analgesia". Obstetrics & Gynecology 27 (4): 472–477. doi:10.1097/00006250-196604000-00003.
- ↑ Carter WJ, Bogert JA (1966). "An effective pre-medication procedure for dental patients". Journal of the Missouri Dental Association 46 (6): 8–9. PMID 5221807.
- ↑ Fuller JD, Crombleholme WR (1987). "Respiratory arrest and prolonged respiratory depression after one low, subcutaneous dose of alphaprodine for obstetric analgesia. A case report". Journal of Reproductive Medicine 32 (2): 149–151. PMID 3560080.