Alkyl polyglycoside
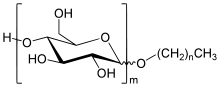
Alkyl polyglycosides (APGs) are a class of non-ionic surfactants widely used in a variety of household and industrial applications. They are derived from sugars, usually glucose derivatives, and fatty alcohols.[1] The raw materials for industrial manufacture are typically starch and fat, and the final products are typically complex mixtures of compounds with different sugars comprising the hydrophilic end and alkyl groups of variable length comprising the hydrophobic end.[2] When derived from glucose, they are known as alkyl polyglucosides.
Uses
APGs are used to enhance the formation of foams in detergents for dishwashing and for delicate fabrics. In addition to their favorable foaming properties, they are attractive because they readily biodegrade.
Preparation
Alkyl glycosides are produced by combining anhydrous glucose or its monohydrate, in the presence of acid catalysts at elevated temperatures. Water released in the reaction mixture is removed from the reaction chamber in the gaseous phase. A partial flow is withdrawn from the liquid reaction mixture and conveyed to a preliminary mixing zone, into which the powdered reactant is introduced simultaneously, where it is processed with the liquid partial flow to a paste and conveyed through a downstream intensive mixer to the reaction chamber. This pressure in the preliminary mixing zone is equalized directly and simultaneously with the reduced pressure in the reaction chamber by the intensive mixer and with atmospheric pressure by the conveying device for the powdered glycose. The consistency of the paste formed in the preliminary mixing zone is chosen so that this paste can be used as a sealant for pressure equalization and hence as a so-called "living seal".
References
- ↑ Karlheinz Hill, Wolfgang von Rybinski, Gerhard Stoll, ed. (2008). Alkyl Polyglycosides. Wiley-VCH. ISBN 978-3-527-61468-4.
- ↑ Iglauer, S. and Wu, Y. and Shuler, P. and Tang, Y. and Goddard, William A., III (2010). "Analysis of the Influence of Alkyl Polyglycoside Surfactant and Cosolvent Structure on Interfacial Tension in Aqueous Formulations versus n-Octane". Tenside Surfactants Detergents 47 (2): 87–97.