Alginic acid
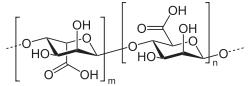 | |
| Names | |
|---|---|
| Other names
Alginic acid, E400 | |
| Identifiers | |
| 9005-32-7 | |
| EC Number | 232-680-1 |
| UNII | 8C3Z4148WZ |
| Properties | |
| (C6H8O6)n | |
| Molar mass | 10,000 – 600,000 |
| Appearance | white to yellow, fibrous powder |
| Density | 1.601 g/cm3 |
| Acidity (pKa) | 1.5–3.5 |
| Pharmacology | |
| ATC code | A02 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |

Alginic acid, also called algin or alginate, is an anionic polysaccharide distributed widely in the cell walls of brown algae, where through binding with water it forms a viscous gum. Its colour ranges from white to yellowish-brown. It is sold in filamentous, granular or powdered forms.
Structure
Alginic acid is a linear copolymer with homopolymeric blocks of (1-4)-linked β-D-mannuronate (M) and its C-5 epimer α-L-guluronate (G) residues, respectively, covalently linked together in different sequences or blocks. The monomers can appear in homopolymeric blocks of consecutive G-residues (G-blocks), consecutive M-residues (M-blocks) or alternating M and G-residues (MG-blocks).
Forms
Alginates are refined from brown seaweeds. A wide variety of brown seaweeds of the phylum Phaeophyceae are harvested throughout the world to be converted into the raw material commonly known as sodium alginate. Sodium alginate has a wide use across a wide variety of industries including food, textile printing and pharmaceutical. Dental impression material utilizes alginate as its means of gelling. Alginate is both food and skin safe.
Seaweeds can be classified into three broad groups based on pigmentation: brown, red and green. These broad groups are the Phaeophyceae, Rhodophyceae and Chlorophyceae, respectively. Brown seaweeds are usually large, and range from the giant kelp Macrocystis pyrifera that is often 20 m long, to thick, leather-like seaweeds from 2–4 m long, to smaller species 30–60 cm long. None of the usual seaweeds for alginate production are cultivated. They cannot be grown by vegetative means, but must go through a reproductive cycle involving an alternation of generations. This makes cultivated brown seaweeds too expensive when compared to the costs of harvesting and transporting wild seaweeds. The only exception is for Laminaria japonica, which is cultivated in China for food but the surplus material is diverted to the alginate industry in China.
Alginates from different species of brown seaweed often have variations in their chemical structure, resulting in different physical properties. For example, some may yield an alginate that gives a strong gel, another a weaker gel; one may readily give a cream/white alginate, another may give that only with difficulty and is best used for technical applications where color does not matter.[1]
Commercial varieties of alginate are extracted from seaweed, including the giant kelp Macrocystis pyrifera, Ascophyllum nodosum, and various types of Laminaria. It is also produced by two bacterial genera Pseudomonas and Azotobacter, which played a major role in the unravelling of its biosynthesis pathway. Bacterial alginates are useful for the production of micro- or nanostructures suitable for medical applications.[2]
The chemical compound sodium alginate is the sodium salt of alginic acid. Its empirical formula is NaC6H7O6. Sodium alginate is a gum, extracted from the cell walls of brown algae.
Potassium alginate is a chemical compound that is the potassium salt of alginic acid. It is an extract of seaweed. Its empirical chemical formula is KC6H7O6.
Calcium alginate, made from sodium alginate from which the sodium salt has been removed and replaced with calcium, has the chemical formula C12H14CaO12.
Production
The processes for the manufacture of sodium alginate from brown seaweeds fall into two categories: 1) Calcium alginate method and, 2) Alginic acid method. The chemistry of the processes used to make sodium alginate from brown seaweeds is relatively simple. The difficulties of the processes arise from the physical separations which are required, such as the need to filter slimy residues from viscous solutions or to separate gelatinous precipitates which hold large amounts of liquid within the structure and which resist filtration and centrifugation.[3]
Uses
Alginate absorbs water quickly, which makes it useful as an additive in dehydrated products such as slimming aids, and in the manufacture of paper and textiles. It is also used for waterproofing and fireproofing fabrics, as a gelling agent, and for thickening drinks, ice cream and cosmetics.
Alginate is used as an ingredient in various pharmaceutical preparations, such as Gaviscon, in which it combines with bicarbonate to inhibit reflux. Alginate is used extensively as an impression-making material in dentistry, prosthetics, lifecasting and occasionally for creating positives for small-scale casting. It is also used in the food industry for thickening soups and jellies.
Calcium alginate is used in different types of medical products including skin wound dressings to promote healing[4] and can be removed with less pain than conventional dressings.
Sodium alginate
Applications for sodium alginate include reactive dye printing and as a thickener for reactive dyes in textile screen-printing. Alginates do not react with these dyes and wash out easily, unlike starch-based thickeners.
As a food additive, sodium alginate is used especially in the production of gel-like foods.
See also
- Hyaluronic acid: a similar polysaccharide in animals.
- Agar
References
- ↑ FAO FISHERIES TECHNICAL PAPER 441,Tevita Bainiloga Jnr, School of Chemistry, University College, University of New South Wales and Australian Defence Force Academy Canberra Australia
- ↑ Remminghorst and Rehm (2009). "Microbial Production of Alginate: Biosynthesis and Applications". Microbial Production of Biopolymers and Polymer Precursors. Caister Academic Press. ISBN 978-1-904455-36-3.
- ↑ FAO Fisheries Technical Paper, 2003
- ↑ Lansdown AB (2002). "Calcium: a potential central regulator in wound healing in the skin". Wound Repair Regen 10 (5): 271–85. PMID 12406163.
External links
- More details about Alginate. Alginate manufacturer KIMICA
- Alginate seaweed sources
- Alginate properties
- article Wired on Easy Cheese, describing sodium alginate
| ||||||||||||||||||||||||||||||