Aldehyde ferredoxin oxidoreductase
| Aldehyde ferredoxin oxidoreductase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.2.7.5 | ||||||||
| CAS number | 138066-90-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
| AFOR_N | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 structure of a hyperthermophilic tungstopterin enzyme, aldehyde ferredoxin oxidoreductase | |||||||||
| Identifiers | |||||||||
| Symbol | AFOR_N | ||||||||
| Pfam | PF02730 | ||||||||
| InterPro | IPR013983 | ||||||||
| SCOP | 1aor | ||||||||
| SUPERFAMILY | 1aor | ||||||||
| |||||||||
| AFOR_C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | AFOR_C | ||||||||
| Pfam | PF01314 | ||||||||
| InterPro | IPR001203 | ||||||||
| SCOP | 1aor | ||||||||
| SUPERFAMILY | 1aor | ||||||||
| |||||||||
In enzymology, an aldehyde ferredoxin oxidoreductase (EC 1.2.7.5) is an enzyme that catalyzes the chemical reaction
- an aldehyde + H2O + 2 oxidized ferredoxin
 an acid + 2 H+ + 2 reduced ferredoxin
an acid + 2 H+ + 2 reduced ferredoxin
This enzyme belongs to the family of oxidoreductases, specifically those acting on the aldehyde or oxo group of donor with an iron-sulfur protein as acceptor. The systematic name of this enzyme class is aldehyde:ferredoxin oxidoreductase. This enzyme is also called AOR. It is a relatively rare example of a tungsten-containing protein.[1]
Occurrence
The active site of the AOR family feature an oxo-tungstern center bound to a pair of molybdopterin cofactors (which does not contain molybdenum) and an 4Fe4S cluster.[2][3] This family includes AOR, formaldehyde ferredoxin oxidoreductase (FOR), glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR), all isolated from hyperthermophilic archea;[2] carboxylic acid reductase found in clostridia;[4] and hydroxycarboxylate viologen oxidoreductase from Proteus vulgaris, the sole member of the AOR family containing molybdenum.[5] GAPOR may be involved in glycolysis,[6] but the functions of the other proteins are not yet clear. AOR has been proposed to be the primary enzyme responsible for oxidising the aldehydes that are produced by the 2-keto acid oxidoreductases.[7]
AOR is found in hyperthermophillic archaea, Pyrococcus furiosus.[1] The archaeons Pyrococcus ES-4 strain and Thermococcus ES-1 strain differ by their substrate specificity: AFOs show a broader size range of its aldehyde subtrates. Its primary role is to oxidize aldehyde coming derived from the metabolsm of amino acids and glucoses.[8] Aldehyde Ferredoxin Oxidoreductase is a member of an AOR family, which includes glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR) and Formaldehyde Ferredoxin Oxidoreductase.[3]
Function
AOR functions at high temperature conditions (~80 degrees Celsius) at an optimal pH of 8-9. It is oxygen-sensitive as it loses bulk of its activity from oxygen exposure and works in the cytoplasm where it is a reducing environment. Thus, either exposure to oxygen or lowering of the temperature causes an irreversible loss of its catalytic properties. Also, as a result of oxygen sensitivity of AOR, purification of the enzyme is done under anoxic environments.[8]
It is proposed that AOR has a role in the Entner-Doudoroff pathway (glucose degradation) due to its increased activity with maltose incorporation.[3] However, other proposals include its role in oxidation of amino acid metabolism aldehyde side products coming from de-aminated 2-ketoacids. The main substrates for aldehyde ferredoxin oxidoreductase are acetaldehyde, phenylacetaldehyde, and isovalerdehyde, which is a metabolic product from common amino acids and glucose.[8] For example, acetaldehyde reaches its kcat/KM value up to 22.0 μM-1s-1.[8] Some bacteria in fact only makes use of amino acids as its carbon sources, such as Thermococcus strain ES1; thus, they utilize aldehyde ferredoxin oxidoreductase to metabolize the amino acid carbon source.[8]
Structure
AOR is homodimeric. Each 67kDa subunit contains 1 tungsten and 4-5 Iron atoms.[3] The two subunits are bridged by a low spin Iron center. It is believed that the two subunits function independently.[3]
- Tungsten-pterin
Tungsten in the active site of AOR adopts a distorted square pyramidal geometry bound an oxo/hydroxo ligand and the dithiolene substituents of two molybdopterin cofactors.[3]
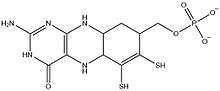
Two molybdopterin cofactors bind tungsten,[9] as observed in many related enzymes.[9] Tungsten is not bonded directly to the protein.[9] Phosphate centers pendant on the cofactor are bound to a Mg2+, which is also bound by Asn93 and Ala183 to complete its octahedral coordination sphere.[3][9] Thus, pterin and Tungsten atoms are connected to the AOR enzyme primarily through pterin's Hydrogen bonding networks with the amino acid residues.[3] In addition, two water ligands that occupy the octahedral geometry take part in hydrogen bonding networks with pterin, phosphate, and Mg2+.[9] While [Fe4S4] cluster is bound by four Cys ligands, Pterin - rich in amino and ether linkages - interacts with the Asp-X-X-Gly-Leu-(Cys/Asp) sequences in the AOR enzyme.[3] In such sequence, Cys494 residue is also hydrogen bonded to the [Fe4S4] cluster.[3] This indicates that Cys494 residue connects the Tungsten site and the [Fe4S4] cluster site in the enzyme.[3] Iron atom in the cluster is additionally bound by three other Cystein ligands: .[9] Also, another linker amino acid residue between ferredoxin cluster and pterin is the Arg76, which hydrogen bonds to both pterin and ferredoxin.[3] It is proposed that such hydrogen bonding interactions imply pterin cyclic ring system as an electron carrier.[3] Additionally the C=O center of the pterin binds Na+.[8] The W=O center is proposed, not verified crystallographically.[9]
AOR consists of three domains, domain 1, 2, and 3.[8] While domain 1 contains pterin bound to tungsten, the other two domains provide a channel from tungsten to protein's surface (15 Angstroms in length) in order to allow specific substrates to enter the enzyme through its channel.[8] In the active site, this pterin molecules is in a saddle-like conformation (500 to the normal plane) to “sit” on the domain 1 which also takes on a form with beta sheets to accommodate the Tungsten-Pterin site.[8]
- Iron
The iron center in between the two subunits serve a structural role in AOR.[8] Iron metal atoms takes on a tetrahedral conformation while the ligand coordination comes from two histidines and glutamic acids.[8] This is not known to have any functional role in the redox activity of the protein.[8]
- Fe4S4 centre
[Fe4S4] cluster in AOR is different in some aspects to other ferredoxin molecules.[3] EPR measurements confirm that it serves as a one-electron shuttle.[3]
Aldehyde ferredoxin oxidoreductase mechanism
In the catalytic cycle, W(VI) (tungsten "six") converts to W(IV) concomitant with oxidation of the aldehyde to a carboxylic acid (equivalently, a carboxylate).[3] A W(V) intermediate can be detected by EPR spectroscopy.[3][8]
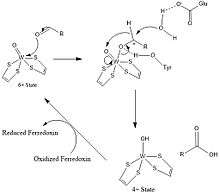
General Reaction Mechanism of AOR:[10]
- RCHO + H2O → RCO2H + 2H+ + 2 e-
The redox equivalents are provided by the 4Fe-4S cluster.
A tyrosine residue is proposed to activate the electrophilic centre of aldehydes by H-bonding to the carbonyl oxygen atom, coordinated to the W centre.[10] A glutamic acid residue near the active site activates a water molecule for a nucleophilic attack on aldehyde carbonyl center.[10] After nucleophilic attack by water, hydride is transferred to oxo-tungsten sie thus, .[10] Subsequently, W(VI) is regenerated by electron transfer to the 4Fe-4S center.[10] With formaldehyde ferredoxin oxidoreductase, Glu308 and Tyr 416 would be involved while Glu313 and His448 is shown to be present in AOR active site.[9][10]
References
- 1 2 Majumdar A, Sarkar S (May 2011). "Bioinorganic chemistry of molybdenum and tungsten enzymes: A structural–functional modeling approach". Coordination Chemistry Reviews 255 (9-10): 1039–1054. doi:10.1016/j.ccr.2010.11.027.
- 1 2 Kisker C, Schindelin H, Rees DC (1997). "Molybdenum-cofactor-containing enzymes: structure and mechanism". Annu. Rev. Biochem. 66: 233–67. doi:10.1146/annurev.biochem.66.1.233. PMID 9242907.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Kletzin A, Adams MW (March 1996). "Tungsten in biological systems". FEMS Microbiol. Rev. 18 (1): 5–63. doi:10.1111/j.1574-6976.1996.tb00226.x. PMID 8672295.
- ↑ White H, Strobl G, Feicht R, Simon H (September 1989). "Carboxylic acid reductase: a new tungsten enzyme catalyses the reduction of non-activated carboxylic acids to aldehydes". Eur. J. Biochem. 184 (1): 89–96. doi:10.1111/j.1432-1033.1989.tb14993.x. PMID 2550230.
- ↑ Trautwein T, Krauss F, Lottspeich F, Simon H (June 1994). "The (2R)-hydroxycarboxylate-viologen-oxidoreductase from Proteus vulgaris is a molybdenum-containing iron-sulphur protein". Eur. J. Biochem. 222 (3): 1025–32. doi:10.1111/j.1432-1033.1994.tb18954.x. PMID 8026480.
- ↑ Mukund S, Adams MW (April 1995). "Glyceraldehyde-3-phosphate ferredoxin oxidoreductase, a novel tungsten-containing enzyme with a potential glycolytic role in the hyperthermophilic archaeon Pyrococcus furiosus". J. Biol. Chem. 270 (15): 8389–92. doi:10.1074/jbc.270.15.8389. PMID 7721730.
- ↑ Ma K, Hutchins A, Sung SJ, Adams MW (September 1997). "Pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon, Pyrococcus furiosus, functions as a CoA-dependent pyruvate decarboxylase". Proc. Natl. Acad. Sci. U.S.A. 94 (18): 9608–13. doi:10.1073/pnas.94.18.9608. PMC 23233. PMID 9275170.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Roy R, Dhawan IK, Johnson MK, Rees DC, Adams MW (2006-04-15). Handbook of Metalloproteins: Aldehyde Ferredoxin Oxidoreductase (5 ed.). John Wiley & Sons, Ltd.
- 1 2 3 4 5 6 7 8 Kisker C, Schindelin H, Rees DC (1997). "Molybdenum-cofactor-containing enzymes: structure and mechanism". Annual Review of Biochemistry 66: 233–67. doi:10.1146/annurev.biochem.66.1.233. PMID 9242907.
- 1 2 3 4 5 6 Bevers LE, Hagedoorn PL, Hagen WR (February 2009). "The bioinorganic chemistry of tungsten". Coordination Chemistry Reviews 253 (3-4): 269–290. doi:10.1016/j.ccr.2008.01.017.
Further reading
- Mukund S, Adams MW (1991). "The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase. Evidence for its participation in a unique glycolytic pathway". J. Biol. Chem. 266 (22): 14208–16. PMID 1907273.
- Johnson JL, Rajagopalan KV, Mukund S, Adams MW (1993). "Identification of molybdopterin as the organic component of the tungsten cofactor in four enzymes from hyperthermophilic Archaea". J. Biol. Chem. 268 (7): 4848–52. PMID 8444863.
- Roy R, Menon AL, Adams MW (2001). "Aldehyde oxidoreductases from Pyrococcus furiosus". Methods Enzymol. 331: 132–44. doi:10.1016/S0076-6879(01)31052-2. PMID 11265456.
| ||||||||||||||||||||||
| ||||||||||||||||||
This article incorporates text from the public domain Pfam and InterPro IPR013983