Acid catalysis
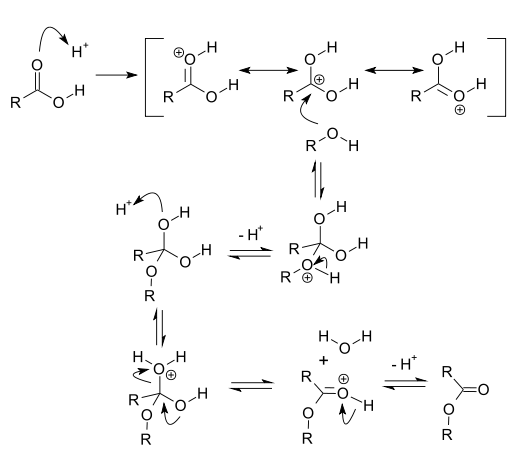
In acid catalysis and base catalysis a chemical reaction is catalyzed by an acid or a base. The acid is the proton donor and the base is the proton acceptor. Typical reactions catalyzed by proton transfer are esterfications and aldol reactions. In these reactions the conjugate acid of the carbonyl group is a better electrophile than the neutral carbonyl group itself. Catalysis by either acid or base can occur in two different ways: specific catalysis and general catalysis.
Use in synthesis
Acid catalysis is mainly used for organic chemical reactions. There are many possible chemical compounds that can act as sources for the protons to be transferred in an acid catalysis system. A compound such as sulfuric acid, H2SO4, can be used. Usually this is done to create a more likely leaving group, such as converting an OH group to a H2O+ group, which can then be eliminated as water (H2O). Acids specifically used for acid catalysis include hydrofluoric acid (in the alkylation process), phosphoric acid, toluenesulfonic acid, polystyrene sulfonate, heteropoly acids, zeolites and graphene oxide.
With carbonyl compounds such as esters, synthesis and hydrolysis go through a tetrahedral transition state, where the central carbon has an oxygen, an alcohol group, and the original alkyl group. Strong acids protonate the carbonyl, which makes the oxygen positively charged, so that it can easily receive the double bond electrons when the alcohol attacks the carbonyl carbon. This enables ester synthesis and hydrolysis. The reaction is an equilibrium between the ester and its cleavage to carboxylic acid and alcohol. On the contrary, strong bases deprotonate the attacking alcohol or amine, which also promotes the reaction. However, bases also deprotonate the acid, which is irreversible. Therefore, in a strongly basic, aqueous environment, esters only hydrolyze.
Solid acid catalysts
In industrial scale chemistry, many processes are catalysed by "solid acids." As heterogeneous catalysts, solid acids do not dissolve in the reaction medium. Well known examples include zeolites, alumina, and various other metal oxides. Such acids are used in cracking. A particularly large scale application is alkylation, e.g. the combination of benzene and ethylene to give ethylbenzene.[1] Many alkylamines are prepared by amination of alcohols.

Kinetics
Specific catalysis
In specific acid catalysis taking place in solvent S, the reaction rate is proportional to the concentration of the protonated solvent molecules SH+.[2] The acid catalyst itself (AH) only contributes to the rate acceleration by shifting the chemical equilibrium between solvent S and AH in favor of the SH+ species.
- S + AH → SH+ + A−
For example in an aqueous buffer solution the reaction rate for reactants R depends on the pH of the system but not on the concentrations of different acids.
This type of chemical kinetics is observed when reactant R1 is in a fast equilibrium with its conjugate acid R1H+ which proceeds to react slowly with R2 to the reaction product; for example, in the acid catalysed aldol reaction.
General catalysis
In general acid catalysis all species capable of donating protons contribute to reaction rate acceleration.[3] The strongest acids are most effective. Reactions in which proton transfer is rate-determining exhibit general acid catalysis, for example diazonium coupling reactions.
![\text{rate}= -\frac{\text{d}[R 1]}{\text{d}t} = k_1[SH^+] [R 1] [R 2] + k_2[AH^1] [R 1] [R 2] + k_3[AH^2] [R 1] [R 2] + ...](../I/m/059dcf7f739042cb999bf9139b20f5e7.png)
When keeping the pH at a constant level but changing the buffer concentration a change in rate signals a general acid catalysis. A constant rate is evidence for a specific acid catalyst.
References
- ↑ Michael Röper, Eugen Gehrer, Thomas Narbeshuber, Wolfgang Siegel "Acylation and Alkylation" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000. doi:10.1002/14356007.a01_185
- ↑ "IUPAC Compendium of Chemical Terminology, 2nd Edition (1997)". www.iupac.org. Retrieved 2009-11-22.
- ↑ "IUPAC Compendium of Chemical Terminology, 2nd Edition (1997)". www.iupac.org. Retrieved 2009-11-22.
![\text{rate}= -\frac{\text{d}[R 1]}{\text{d}t} = k[SH^+] [R 1] [R 2]](../I/m/4530e4cf5d35dcb7f80285bd1c93399d.png)