Acetylenedicarboxylic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
But-2-ynedioic acid | |
| Other names
2-Butynedioic acid | |
| Identifiers | |
| 142-45-0 | |
| ChEBI | CHEBI:30781 |
| ChemSpider | 362 |
| Jmol interactive 3D | Image Image |
| KEGG | C03248 |
| PubChem | 371 |
| |
| |
| Properties | |
| C4H2O4 | |
| Molar mass | 114.06 g·mol−1 |
| Appearance | Crystalline solid |
| Melting point | 175 to 176 °C (347 to 349 °F; 448 to 449 K) (decomposes)[2] 180–187 °C (decomposes)[1] |
| Hazards | |
| R-phrases | R25 R36/37/38 |
| S-phrases | S26 S45 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
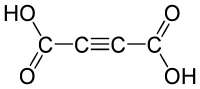
Acetylenedicarboxylic acid or butynedioic acid is an organic compound (a dicarboxylic acid) with the formula C4H2O4 or HO2C-C≡C-CO2H. It is a crystalline solid that is soluble in diethyl ether.
The removal of two protons yields the acetylenedicarboxylate dianion C4O42−, which consists only of carbon and oxygen, making it an oxocarbon anion. Partial ionization yields the monovalent hydrogenacetylenedicarboxylate anion HC4O4−.
The acid was first described in 1877 by Polish chemist Ernest Bandrowski.[2][3][4] It can be obtained by treating α,β-dibromosuccinic acid with potassium hydroxide KOH in methanol or ethanol. The reaction yields potassium bromide and potassium acetylenedicarboxylate. The salts are separated and the latter is treated with sulfuric acid.[2]
Acetylenedicarboxylic acid is used in the synthesis of dimethyl acetylenedicarboxylate, an important laboratory reagent. Both the acid and the monobasic salt potassium hydrogenacetylenedicarboxylate KC4HO4 are commonly traded as laboratory chemicals.
See also
References
- 1 2 Acetylenedicarboxylic acid at Sigma-Aldrich
- 1 2 3 Abbott, T. W.; Arnold, R. T.; Thompson, R. B. "Acetylenedicarboxylic acid". Org. Synth.; Coll. Vol. 2, p. 10
- ↑ Bandrowski, E. (1877). "Ueber Acetylendicarbonsäure". Berichte der deutschen chemischen Gesellschaft 10: 838. doi:10.1002/cber.187701001231.
- ↑ E. Bandrowski (1879). "Weitere Beiträge zur Kenntniss der Acetylendicarbonsäure". Berichte der deutschen chemischen Gesellschaft 12 (2): 2212–2216. doi:10.1002/cber.187901202261.