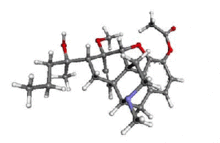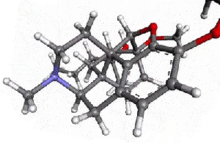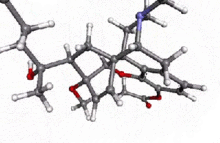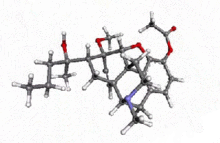
Acetorphine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
4,5α-epoxy-7α-(1-hydroxy-1-methylbutyl)-6-methoxy -17-methyl-6,14-endo-ethenomorphinan-3-yl acetate | |
| Clinical data | |
| Legal status |
|
| Identifiers | |
| CAS Number |
25333-77-1 |
| ATC code | None |
| PubChem | CID 62795 |
| ChemSpider |
16736130 |
| UNII |
2OGQ81529L |
| Chemical data | |
| Formula | C27H35NO5 |
| Molar mass | 453.57 g/mol |
| |
| |
| | |
Acetorphine is a potent opioid analgesic, up to 8700 times stronger than morphine by weight.[1] It is a derivative of the more well-known opioid etorphine, which is used as a very potent veterinary painkiller and anesthetic medication, primarily for the sedation of large animals such as elephants, giraffes and rhinos.
Acetorphine was developed in 1966 by the Reckitt research group that developed etorphine. Acetorphine was developed for the same purpose as etorphine itself, namely as a strong tranquilizer for use in immobilizing large animals in veterinary medicine. Despite showing some advantages over etorphine, for instance producing less toxic side effects in giraffes,[2] acetorphine was never widely adopted for veterinary use, and etorphine (along with other tranquilizers such as carfentanil and azaperone) remains the drug of choice in this application.
Legal Status
United States
Acetorphine is a Schedule I controlled substance in the United States. Its DEA Administrative Controlled Substances Control Number is 9319 and the one salt in use, acetorphine hydrochloride, has a freebase conversion ratio of 0.93.
Australia
Acetorphine is a schedule 9 substance in Australia under the Poisons Standard (October 2015).[3] A schedule 9 drug is outlined in the Poisons Act 1964 as "Substances which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of the CEO." [4]
Under the Misuse of Drugs Act 1981 6.0g is the amount required determining a court of trial, 2.0g is considered intent to sell and supply. [5]
References
- ↑ Bentley, K. W.; Hardy, D. G. (1967). "Novel analgesics and molecular rearrangements in the morphine-thebaine group. 3. Alcohols of the 6,14-endo-ethenotetrahydrooripavine series and derived analogs of N-allylnormorphine and -norcodeine". Journal of the American Chemical Society 89 (13): 3281–3292. doi:10.1021/ja00989a032. PMID 6042764.
- ↑ UNODC (1968). "The case of etorphine and acetorphine". Bulletin on Narcotics (UNODC) 1968 (2): 51–52.
- ↑ Poisons Standard October 2015 https://www.comlaw.gov.au/Details/F2015L01534
- ↑ Poisons Act 1964 http://www.slp.wa.gov.au/pco/prod/FileStore.nsf/Documents/MRDocument:26063P/$FILE/Poisons%20Act%201964%20-%20%5B09-f0-04%5D.pdf?OpenElement
- ↑ Misuse of Drugs Act 1981 (2015) http://www.slp.wa.gov.au/pco/prod/FileStore.nsf/Documents/MRDocument:28280P/$FILE/Misuse%20Of%20Drugs%20Act%201981%20-%20%5B06-e0-00%5D.pdf?OpenElement
See also
External links
- Space filling 3D model and ball & stick 3D model full motion animated rotating of Acetorphine/Acetyletorphine
- 2D nonanimated/nonmoving rendering of Acetorphine/Acetyletorphine as a flat diagram