Acalabrutinib
 | |
| Systematic (IUPAC) name | |
|---|---|
|
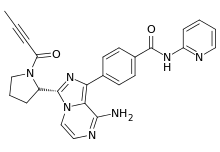
4-{8-Amino-3-[(2S)-1-(2-butynoyl)-2-pyrrolidinyl]imidazo[1,5-a]pyrazin-1-yl}-N-(2-pyridinyl)benzamide | |
| Identifiers | |
| CAS Number | 1420477-60-6 |
| ChemSpider | 36764951 |
| Chemical data | |
| Formula | C26H23N7O2 |
| Molar mass | 465.507 |
| |
| |
Acalabrutinib, (rINN,[1] ACP-196) is a 2nd generation BTK inhibitor[2] developed by Acerta Pharma.[3]
It is designed to be more selective (fewer side-effects) than ibrutinib.[2]
As of 2015 it is in late stage clinical trials for relapsed chronic lymphocytic leukemia. Interim results are encouraging : 95% overall response rate.[2][4]
It is also in another 20 clinical trials (alone and in combination) for various cancers.[4][5]
References
- ↑ "WHO Drug Information - recommended INN" (PDF). WHO Drug Information. World Health Oorganisation. Retrieved 24 December 2015.
- 1 2 3 Byrd; et al. (2015). "Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia.". doi:10.1056/NEJMoa1509981.
- ↑ "AstraZeneca to buy Acerta for blood cancer drug". www.rsc.org. Chemistry World - Royal Society of Chemistry. Retrieved 24 December 2015.
- 1 2 Acerta Pharma Announces Study Published in New England Journal of Medicine Demonstrates Acalabrutinib (ACP-196) Shows Marked Activity in Relapsed Chronic Lymphocytic Leukemia
- ↑ 21 studies found for: ACP-196
This article is issued from Wikipedia - version of the Monday, February 08, 2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.