ATMP
For other uses, see ATMP (disambiguation).
 | |
| Names | |
|---|---|
| IUPAC name
[Bis(phosphonomethyl)amino]methylphosphonic acid | |
| Other names
Tris(phosphonomethyl)amine; Nitrilotrimethylphosphonic acid; Aminotris(methylphosphonic acid); ATMP; NTMP | |
| Identifiers | |
| 6419-19-8 | |
| ChEMBL | ChEMBL260191 |
| ChemSpider | 15833 |
| EC Number | 229-146-5 |
| Jmol interactive 3D | Image |
| PubChem | 16698 |
| UNII | 1Y702GD0FG |
| |
| |
| Properties | |
| C3H12NO9P3 | |
| Molar mass | 299.05 g·mol−1 |
| Appearance | White solid |
| Density | 1.33 g/cm3 (20 °C) |
| Melting point | 200 °C (392 °F; 473 K) decomposes |
| 61 g/100 mL | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
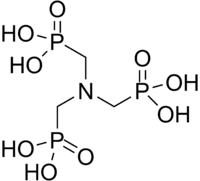
ATMP or aminotris(methylenephosphonic acid) is a phosphonic acid with chemical formula N(CH2PO3H2)3. It has chelating properties. It can be synthesized from the Mannich-type reaction of ammonia, formaldehyde, and phosphorous acid.[1]
Properties
ATMP has better antiscale performance than that of polyphosphate through its excellent chelating ability, low threshold inhibition and lattice distortion process. It can prevent scale formation in water systems. ATMP is the phosphonate analog of nitrilotriacetic acid.
Applications
- Detergents and cleaning agents
- Water treatment
- Scaling inhibition
- Chelation
References
- ↑ Moedritzer, Kurt; Irani, Riyad R. (1966). "The Direct Synthesis of α-Aminomethylphosphonic Acids. Mannich-Type Reactions with Orthophosphorous Acid". The Journal of Organic Chemistry 31 (5): 1603. doi:10.1021/jo01343a067.
This article is issued from Wikipedia - version of the Tuesday, July 07, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.