Actinin, alpha 2
Alpha-actinin 2 is a protein which in humans is encoded by the ACTN2 gene.[1] This gene encodes an alpha-actinin isoform that is expressed in both skeletal and cardiac muscles and functions to anchor myofibrillar actin thin filaments and titin to Z-discs.
Structure
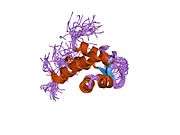
Alpha-actinin 2 is a 103.8 kDa protein composed of 894 amino acids.[2][3] Each molecule is rod-shaped (35 nm in length) and it homodimerizes in an anti-parallel fashion. Each monomer has an N-terminal actin-binding region composed of two calponin homology domains, two C-terminal EF hand domains, and four tandem spectrin-like repeats form the rod domain in the central region of the molecule.[4] The high-resolution crystal structure of human alpha-actinin 2 at 3.5 Å was recently resolved.[5] Alpha actinins belong to the spectrin gene superfamily which represents a diverse group of actin-binding cytoskeletal proteins, including spectrin, dystrophin, utrophin and fimbrin.[4] Skeletal, cardiac, and smooth muscle isoforms are localized to the Z-disc and analogous dense bodies, where they help anchor the myofibrillar actin filaments. Alpha-actinin 2 has been shown to interact with KCNA5,[6][7] DLG1,[6] DISC1,[8] MYOZ1,[9] GRIN2B,[10] ADAM12,[11] ACTN3,[12] MYPN,[13] PDLIM3,[14] PKN,[15] MYOT,[16] TTN,[17] NMDAR,[18] SYNPO2,[19] LDB3,[20] and FATZ.[21]
Function
The primary function of alpha-actinin 2 is to crosslink filamentous actin molecules and titin molecules from adjoining sarcomeres at Z-discs, a function that is modulated by phospholipids.[22][23] It is clear from studies by Hampton et al. that this crosslinking can assume a variety of conformations, with preferences for 60° and 120° angles.[24] Alpha-actinin 2 also functions in docking signalling molecules at Z-discs, and additional studies have also implicated alpha-actinin 2 in the binding of cardiac ion channels, Kv1.5 in particular.[6]
Clinical significance
Mutations in ACTN2 are associated with hypertrophic cardiomyopathy,[25] as well as dilated cardiomyopathy and endocardial fibroelastosis.[26] The diverse functions of alpha-actinin 2 are reflected in the diverse clinical presentation of patients carrying ACTN2 mutations.[27]
References
- ↑ "Entrez Gene: ACTN2 actinin, alpha 2".
- ↑ ""Protein Information – Basic Information: Protein COPaKB ID: P35609". Cardiac Organellar Protein Atlas Knowledgebase.
- ↑ Zong, N. C.; Li, H; Li, H; Lam, M. P.; Jimenez, R. C.; Kim, C. S.; Deng, N; Kim, A. K.; Choi, J. H.; Zelaya, I; Liem, D; Meyer, D; Odeberg, J; Fang, C; Lu, H. J.; Xu, T; Weiss, J; Duan, H; Uhlen, M; Yates Jr, 3rd; Apweiler, R; Ge, J; Hermjakob, H; Ping, P (2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- 1 2 Luther, P. K. (2009). "The vertebrate muscle Z-disc: Sarcomere anchor for structure and signalling". Journal of Muscle Research and Cell Motility 30 (5-6): 171–85. doi:10.1007/s10974-009-9189-6. PMC 2799012. PMID 19830582.
- ↑ Ribeiro Ede Jr, A; Pinotsis, N; Ghisleni, A; Salmazo, A; Konarev, P. V.; Kostan, J; Sjöblom, B; Schreiner, C; Polyansky, A. A.; Gkougkoulia, E. A.; Holt, M. R.; Aachmann, F. L.; Zagrović, B; Bordignon, E; Pirker, K. F.; Svergun, D. I.; Gautel, M; Djinović-Carugo, K (2014). "The structure and regulation of human muscle α-actinin". Cell 159 (6): 1447–60. doi:10.1016/j.cell.2014.10.056. PMC 4259493. PMID 25433700.
- 1 2 3 Eldstrom, Jodene; Choi Woo Sung; Steele David F; Fedida David (Jul 2003). "SAP97 increases Kv1.5 currents through an indirect N-terminal mechanism". FEBS Lett. (Netherlands) 547 (1-3): 205–11. doi:10.1016/S0014-5793(03)00668-9. ISSN 0014-5793. PMID 12860415.
- ↑ Maruoka, N D; Steele D F; Au B P; Dan P; Zhang X; Moore E D; Fedida D (May 2000). "alpha-actinin-2 couples to cardiac Kv1.5 channels, regulating current density and channel localization in HEK cells". FEBS Lett. (NETHERLANDS) 473 (2): 188–94. doi:10.1016/S0014-5793(00)01521-0. ISSN 0014-5793. PMID 10812072.
- ↑ Morris, Jill A; Kandpal Geeta; Ma Lei; Austin Christopher P (Jul 2003). "DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation". Hum. Mol. Genet. (England) 12 (13): 1591–608. doi:10.1093/hmg/ddg162. ISSN 0964-6906. PMID 12812986.
- ↑ Faulkner, G; Pallavicini A, Comelli A, Salamon M, Bortoletto G, Ievolella C, Trevisan S, Kojic' S, Dalla Vecchia F, Laveder P, Valle G, Lanfranchi G (Dec 2000). "FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle". J. Biol. Chem. (UNITED STATES) 275 (52): 41234–42. doi:10.1074/jbc.M007493200. ISSN 0021-9258. PMID 10984498.
- ↑ Wyszynski, M; Lin J; Rao A; Nigh E; Beggs A H; Craig A M; Sheng M (Jan 1997). "Competitive binding of alpha-actinin and calmodulin to the NMDA receptor". Nature (ENGLAND) 385 (6615): 439–42. doi:10.1038/385439a0. ISSN 0028-0836. PMID 9009191.
- ↑ Galliano, M F; Huet C; Frygelius J; Polgren A; Wewer U M; Engvall E (May 2000). "Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha -actinin-2, is required for myoblast fusion". J. Biol. Chem. (UNITED STATES) 275 (18): 13933–9. doi:10.1074/jbc.275.18.13933. ISSN 0021-9258. PMID 10788519.
- ↑ Chan, Y; Tong H Q; Beggs A H; Kunkel L M (Jul 1998). "Human skeletal muscle-specific alpha-actinin-2 and -3 isoforms form homodimers and heterodimers in vitro and in vivo". Biochem. Biophys. Res. Commun. (UNITED STATES) 248 (1): 134–9. doi:10.1006/bbrc.1998.8920. ISSN 0006-291X. PMID 9675099.
- ↑ Bang, M L; Mudry R E; McElhinny A S; Trombitás K; Geach A J; Yamasaki R; Sorimachi H; Granzier H; Gregorio C C; Labeit S (Apr 2001). "Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies". J. Cell Biol. (United States) 153 (2): 413–27. doi:10.1083/jcb.153.2.413. ISSN 0021-9525. PMC 2169455. PMID 11309420.
- ↑ Pomiès, P; MacAlma, T; Beckerle, M. C. (1999). "Purification and characterization of an alpha-actinin-binding PDZ-LIM protein that is up-regulated during muscle differentiation". The Journal of Biological Chemistry 274 (41): 29242–50. doi:10.1074/jbc.274.41.29242. PMID 10506181.
- ↑ Mukai, H; Toshimori, M; Shibata, H; Takanaga, H; Kitagawa, M; Miyahara, M; Shimakawa, M; Ono, Y (1997). "Interaction of PKN with alpha-actinin". The Journal of Biological Chemistry 272 (8): 4740–6. doi:10.1074/jbc.272.8.4740. PMID 9030526.
- ↑ Salmikangas, P; Mykkänen, O. M.; Grönholm, M; Heiska, L; Kere, J; Carpén, O (1999). "Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy". Human Molecular Genetics 8 (7): 1329–36. doi:10.1093/hmg/8.7.1329. PMID 10369880.
- ↑ Young, P; Ferguson, C; Bañuelos, S; Gautel, M (1998). "Molecular structure of the sarcomeric Z-disk: Two types of titin interactions lead to an asymmetrical sorting of alpha-actinin". The EMBO Journal 17 (6): 1614–24. doi:10.1093/emboj/17.6.1614. PMC 1170509. PMID 9501083.
- ↑ Chunn, C. J.; Starr, P. R.; Gilbert, D. N. (1977). "Neutrophil toxicity of amphotericin B". Antimicrobial agents and chemotherapy 12 (2): 226–30. doi:10.1128/aac.12.2.226. PMC 429889. PMID 900919.
- ↑ Linnemann, A; Van Der Ven, P. F.; Vakeel, P; Albinus, B; Simonis, D; Bendas, G; Schenk, J. A.; Micheel, B; Kley, R. A.; Fürst, D. O. (2010). "The sarcomeric Z-disc component myopodin is a multiadapter protein that interacts with filamin and alpha-actinin". European Journal of Cell Biology 89 (9): 681–92. doi:10.1016/j.ejcb.2010.04.004. PMID 20554076.
- ↑ Jani, K; Schöck, F (2007). "Zasp is required for the assembly of functional integrin adhesion sites". The Journal of Cell Biology 179 (7): 1583–97. doi:10.1083/jcb.200707045. PMC 2373490. PMID 18166658.
- ↑ Faulkner, G; Pallavicini, A; Comelli, A; Salamon, M; Bortoletto, G; Ievolella, C; Trevisan, S; Kojic', S; Dalla Vecchia, F; Laveder, P; Valle, G; Lanfranchi, G (2000). "FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle". Journal of Biological Chemistry 275 (52): 41234–42. doi:10.1074/jbc.M007493200. PMID 10984498.
- ↑ Young, P; Gautel, M (2000). "The interaction of titin and alpha-actinin is controlled by a phospholipid-regulated intramolecular pseudoligand mechanism". The EMBO Journal 19 (23): 6331–40. doi:10.1093/emboj/19.23.6331. PMC 305858. PMID 11101506.
- ↑ Fukami, K; Furuhashi, K; Inagaki, M; Endo, T; Hatano, S; Takenawa, T (1992). "Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function". Nature 359 (6391): 150–2. doi:10.1038/359150a0. PMID 1326084.
- ↑ Hampton, C. M.; Taylor, D. W.; Taylor, K. A. (2007). "Novel structures for alpha-actinin:F-actin interactions and their implications for actin-membrane attachment and tension sensing in the cytoskeleton". Journal of Molecular Biology 368 (1): 92–104. doi:10.1016/j.jmb.2007.01.071. PMC 1919418. PMID 17331538.
- ↑ Chiu, C; Bagnall, R. D.; Ingles, J; Yeates, L; Kennerson, M; Donald, J. A.; Jormakka, M; Lind, J. M.; Semsarian, C (2010). "Mutations in alpha-actinin-2 cause hypertrophic cardiomyopathy: A genome-wide analysis". Journal of the American College of Cardiology 55 (11): 1127–35. doi:10.1016/j.jacc.2009.11.016. PMID 20022194.
- ↑ Mohapatra, B; Jimenez, S; Lin, J. H.; Bowles, K. R.; Coveler, K. J.; Marx, J. G.; Chrisco, M. A.; Murphy, R. T.; Lurie, P. R.; Schwartz, R. J.; Elliott, P. M.; Vatta, M; McKenna, W; Towbin, J. A.; Bowles, N. E. (2003). "Mutations in the muscle LIM protein and alpha-actinin-2 genes in dilated cardiomyopathy and endocardial fibroelastosis". Molecular genetics and metabolism 80 (1-2): 207–15. doi:10.1016/s1096-7192(03)00142-2. PMID 14567970.
- ↑ Bagnall, R. D.; Molloy, L. K.; Kalman, J. M.; Semsarian, C (2014). "Exome sequencing identifies a mutation in the ACTN2 gene in a family with idiopathic ventricular fibrillation, left ventricular noncompaction, and sudden death". BMC Medical Genetics 15 (1): 99. doi:10.1186/s12881-014-0099-0. PMID 25224718.
Further reading
- Faulkner G, Lanfranchi G, Valle G (2002). "Telethonin and other new proteins of the Z-disc of skeletal muscle.". IUBMB Life 51 (5): 275–82. doi:10.1080/152165401317190761. PMID 11699871.
- Beggs AH, Byers TJ, Knoll JH; et al. (1992). "Cloning and characterization of two human skeletal muscle alpha-actinin genes located on chromosomes 1 and 11.". J. Biol. Chem. 267 (13): 9281–8. PMID 1339456.
- Beggs AH, Phillips HA, Kozman H; et al. (1992). "A (CA)n repeat polymorphism for the human skeletal muscle alpha-actinin gene ACTN2 and its localization on the linkage map of chromosome 1.". Genomics 13 (4): 1314–5. doi:10.1016/0888-7543(92)90054-V. PMID 1505962.
- Yürüker B, Niggli V (1992). "Alpha-actinin and vinculin in human neutrophils: reorganization during adhesion and relation to the actin network.". J. Cell. Sci. 101 (2): 403–14. PMID 1629252.
- Pavalko FM, LaRoche SM (1993). "Activation of human neutrophils induces an interaction between the integrin beta 2-subunit (CD18) and the actin binding protein alpha-actinin.". J. Immunol. 151 (7): 3795–807. PMID 8104223.
- Yoshida M, Westlin WF, Wang N; et al. (1996). "Leukocyte adhesion to vascular endothelium induces E-selectin linkage to the actin cytoskeleton.". J. Cell Biol. 133 (2): 445–55. doi:10.1083/jcb.133.2.445. PMC 2120789. PMID 8609175.
- Wyszynski M, Lin J, Rao A; et al. (1997). "Competitive binding of alpha-actinin and calmodulin to the NMDA receptor.". Nature 385 (6615): 439–42. doi:10.1038/385439a0. PMID 9009191.
- Mukai H, Toshimori M, Shibata H; et al. (1997). "Interaction of PKN with alpha-actinin.". J. Biol. Chem. 272 (8): 4740–6. doi:10.1074/jbc.272.8.4740. PMID 9030526.
- Young P, Ferguson C, Bañuelos S, Gautel M (1998). "Molecular structure of the sarcomeric Z-disk: two types of titin interactions lead to an asymmetrical sorting of alpha-actinin.". EMBO J. 17 (6): 1614–24. doi:10.1093/emboj/17.6.1614. PMC 1170509. PMID 9501083.
- Zhang S, Ehlers MD, Bernhardt JP; et al. (1998). "Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors.". Neuron 21 (2): 443–53. doi:10.1016/S0896-6273(00)80553-X. PMID 9728925.
- Krupp JJ, Vissel B, Thomas CG; et al. (1999). "Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors.". J. Neurosci. 19 (4): 1165–78. PMID 9952395.
- Zhou Q, Ruiz-Lozano P, Martone ME, Chen J (1999). "Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to alpha-actinin-2 and protein kinase C.". J. Biol. Chem. 274 (28): 19807–13. doi:10.1074/jbc.274.28.19807. PMID 10391924.
- Faulkner G, Pallavicini A, Formentin E; et al. (1999). "ZASP: a new Z-band alternatively spliced PDZ-motif protein.". J. Cell Biol. 146 (2): 465–75. doi:10.1083/jcb.146.2.465. PMC 3206570. PMID 10427098.
- Tiso N, Majetti M, Stanchi F; et al. (1999). "Fine mapping and genomic structure of ACTN2, the human gene coding for the sarcomeric isoform of alpha-actinin-2, expressed in skeletal and cardiac muscle.". Biochem. Biophys. Res. Commun. 265 (1): 256–9. doi:10.1006/bbrc.1999.1661. PMID 10548523.
- Nikolopoulos SN, Spengler BA, Kisselbach K; et al. (2000). "The human non-muscle alpha-actinin protein encoded by the ACTN4 gene suppresses tumorigenicity of human neuroblastoma cells.". Oncogene 19 (3): 380–6. doi:10.1038/sj.onc.1203310. PMID 10656685.
- Galliano MF, Huet C, Frygelius J; et al. (2000). "Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha -actinin-2, is required for myoblast fusion.". J. Biol. Chem. 275 (18): 13933–9. doi:10.1074/jbc.275.18.13933. PMID 10788519.
- Maruoka ND, Steele DF, Au BP; et al. (2000). "alpha-actinin-2 couples to cardiac Kv1.5 channels, regulating current density and channel localization in HEK cells.". FEBS Lett. 473 (2): 188–94. doi:10.1016/S0014-5793(00)01521-0. PMID 10812072.
- Kotaka M, Kostin S, Ngai S; et al. (2000). "Interaction of hCLIM1, an enigma family protein, with alpha-actinin 2.". J. Cell. Biochem. 78 (4): 558–65. doi:10.1002/1097-4644(20000915)78:4<558::AID-JCB5>3.0.CO;2-I. PMID 10861853.
- Parast MM, Otey CA (2000). "Characterization of palladin, a novel protein localized to stress fibers and cell adhesions.". J. Cell Biol. 150 (3): 643–56. doi:10.1083/jcb.150.3.643. PMC 2175193. PMID 10931874.
- Faulkner G, Pallavicini A, Comelli A; et al. (2001). "FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle.". J. Biol. Chem. 275 (52): 41234–42. doi:10.1074/jbc.M007493200. PMID 10984498.
External links
- Mass spectrometry characterization of human ACTN2 at COPaKB
- GeneReviews/NIH/NCBI/UW entry on Familial Hypertrophic Cardiomyopathy Overview
| |||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||