7-Keto-DHEA
 7-Keto-DHEA | |
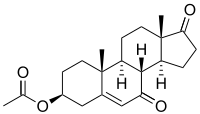 7-Keto-DHEA acetate | |
| Names | |
|---|---|
| IUPAC name
(3β)-3-Hydroxyandrost-5-ene-7,17-dione | |
| Other names
7-Keto; 7-Oxo-DHEA; 7-Ketodehydroepiandrosterone; 7-Oxodehydroepiandrosterone; 3β-Hydroxy-5-androstene-7,17-dione; 5-Androsten-3β-ol-7,17-dione | |
| Identifiers | |
| 566-19-8 1449-61-2 (acetate) | |
| ChemSpider | 167751 |
| Jmol interactive 3D | Image |
| PubChem | 193313 |
| |
| |
| Properties | |
| C19H26O3 | |
| Molar mass | 302.41 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
7-Keto-DHEA (also known as 7-Keto, 7-ketodehydroepiandrosterone, or 7-oxodehydroepiandrosterone)[1] is a steroid produced by metabolism of the prohormone dehydroepiandrosterone (DHEA).[2] 7-Keto is not directly converted to testosterone or estrogen, and has thus been investigated as a potentially more useful relative of DHEA.[3][4] It is often used as the acetate ester prodrug 7-Keto-DHEA acetate.
While in vitro and animal studies have suggested potential uses for 7-Keto in humans, there is currently insufficient scientific evidence to support its use as a weight-loss aid, muscle-builder, immune stimulant, or for any other clinical use.[5] Nevertheless, 7-Keto is marketed as a dietary supplement with the implication that it may accelerate weight loss, increase metabolism, enhance memory, or prevent age-related changes. When used in a topical (skin lotion) product 7-Keto caused long-lasting changes in the body's levels of testosterone, epitestosterone, estradiol, and other steroid hormones.[6] Researchers have raised concern that supplements may trigger positive tests for performance-enhancing drugs.[7][8]
The World Anti-Doping Agency lists 7-keto DHEA as a prohibited anabolic agent.[9]
Effects and uses
7-Keto is unique among other derivatives of DHEA because of its oxygenated 7-position. This molecular configuration imparts different characteristics to the molecule, and research reveals that 7-Keto DHEA has multiple distinct effects in the body.
Increased metabolism/weight loss
7-Keto demonstrates documented thermogenic activity in rats. This is accomplished through the activation of three thermogenic enzymes: Glycerol-3-Phosphate Dehydrogenase, Malic Enzyme and Fatty Acyl CoA Oxidase.[3][10][11][12] In keeping with the biological definition of thermogenesis, all three of these enzyme activations drive energy-producing substrates in a direction of less efficient ATP production relative to heat production. The enzymes also promote the utilization of fat stores for energy and heat production. This is the basis for 7-Keto’s ability to enhance thermogenesis and, through that mechanism, accelerate the utilization of fat stores for energy.
A 2007 study demonstrated that administration of 7-Keto to overweight adults in conjunction with a calorie-restricted diet effectively reverses the decline in resting metabolic rate (RMR) normally associated with dieting. 7-Keto demonstrated an ability to increase RMR by 1.4% above baseline levels and demonstrated a 5.4% increase in daily RMR when administered with a calorie-restricted diet.[13]
7-Keto achieves this thermogenic effect without cardiovascular or central nervous system side effects, which are commonly seen with stimulant-associated thermogenic agents.[4][13]
Age-related immune enhancement
Although the aging process affects all of the segments of the immune system, investigators have identified abnormalities in the cellular or T-cell mediated immune function in the elderly.[14] The decline in T-cell immune function is associated with an increased susceptibility to infections.[14] For example, individuals with age-related declines in cellular immunity have an impaired response to influenza vaccine, making them more susceptible to getting the “flu” even though they have had their flu shot.
In a clinical study presented at the Federation of American Societies for Experimental Biology meeting in April 2004, the effect of 7-Keto was evaluated in regard to its effect on elderly immune function.
Safety
7-Keto has been the subject of a series of toxicological evaluations. These studies include: AMES Mutagenicity Test, Acute Oral Dose LD50 in Rats, Escalating Dose Oral Gavage in Rhesus Monkeys and 28 Day Oral Gavage in Rhesus Monkeys.[15][16]
There were no adverse effects in any of these studies. This pre-clinical work was followed by a Phase I safety study in humans performed by Davidson and colleagues at the Chicago Center for Clinical Research.[4] This study was published in Clinical Investigative Medicine and indicated that 7-Keto was safe for human consumption at doses up to 200 mg per day for 4 weeks. As in the toxicological studies, there were no serious adverse reactions or hormone related side effects reported. Data on the safety of long-term use (beyond 4 weeks) are lacking.[4]
In addition, a complete pharmacokinetic analysis was completed as part of this study. This pharmacokinetic analysis describes exactly how the body absorbs, metabolizes, distributes and excretes 7-Keto. It reveals that 7-Keto is rapidly absorbed and converted to its sulfate derivative, it reaches peak plasma concentrations in 2.2 hours and has a half-life of 2.17 hours and there is no accumulation with repeated dosing.[4]
To date, there have been two pre-market notifications filed with the FDA announcing intent to market 7-Keto as a dietary ingredient. These notifications had to demonstrate to FDA’s satisfaction that there is no issue with the safety of the subject ingredient. The FDA had no objection, in each of these notifications, to the marketing and sale of 7-Keto.[17]
Pharmacokinetics
Similar to DHEA, 7-Keto is rapidly sulfated to 7-Keto -sulfate in the body. An analytical method was developed for quantification of 7-Keto -sulfate in human plasma. This was an HPLC method, which utilized calibration curves for 7-Keto-sulfate in the range of 10 to 500 ng/ml.
Trough levels were measured after each escalating dose sequence; 0, 50, 100 and 200 mg per day. Trough plasma concentrations increased proportionally to the daily dose. Mean trough levels (15.8 ng/ml) after 1 week of dosing at 200 mg/day were similar to those determined after 4 weeks of dosing (16.3 ng/ml). This indicated that the ratio of the formation rate of this metabolite to its elimination clearance is constant during multiple dosing and does not accumulate.
After a twelve-hour washout period, all 22 subjects were given a single dose of 7-Keto at 100 mg and plasma levels were obtained at 0.25, 0.50, 1.0, 2.0, 4.0, 6.0 and 12.0 hrs after the dose. The mean plasma concentrations as measured in the study demonstrated a peak plasma level of 158 ng/ml, which occurred at 2.2 hours after the dose. The average elimination half-life was determined to be 2.17 hours. Based on the data, the dosing regime of twice per day was recommended as the ideal dosing schedule with steady state blood levels being the goal.
A one-compartment model was assumed with first order absorption and no lag phase. The results of these simulations using this pharmacokinetic model showed that there was good agreement between: a) the simulated and measured means of the trough plasma levels, and b) the plasma concentrations of a single 100 mg oral dose.
The pharmacokinetic analysis revealed that 7-Keto is rapidly absorbed and converted to its sulfate derivative, it reaches peak plasma concentrations in 2.2 hours, and it has a half-life of 2.17 hours. There is no accumulation with repeated dosing and, with twice daily dosing, should reach a steady state plasma level in 11 hours.
History
To search for possible metabolites of DHEA that might have greater biological activity, greater specificity, and fewer propensities to form sex hormones, Dr. Lardy initiated a program assaying the derivatives of DHEA. The activity of 150 of these metabolites was monitored by measuring the induction of two thermogenic enzymes, mitochondrial glycerol-3-phosphate dehydrogenase and cytosolic malic enzyme. The results of this landmark study were published in the journal Steroids in 1998[3] and revealed that many of these steroids did not induce the activity of these thermogenic enzymes, whereas the 7-Keto metabolite did. In fact, 7-Keto was 2.5 times more active than DHEA at inducing the activity of these thermogenic enzymes. In later work by Marenich,[18] it was discovered that the urinary excretion of 7-Keto declines with age in a similar manner to its parent compound, DHEA. Based on these discovered advantages, the 7-Keto metabolite was chosen for further study as a weight loss ingredient.
Society and culture
7-Keto is legal to sell in the United States as a dietary supplement. It currently has two premarket notifications with the FDA.[17] Commercial sales of 7-Keto as a dietary ingredient began in June 1998.
References
- ↑ This compound has a number of chemical names, including:
- 7-Ketodehydroepiandrosterone
- 7-Oxodehydroepiandrosterone
- 3β-Hydroxy-5-androstene-7,17-dione
- 5-Androsten-3β-ol-7,17-dione
- 3β-Acetoxyandrost-5-ene-7,17-dione
- 7-Oxo-dehydroepiandrosterone acetate
- 7-Oxo DHEA acetate
- 3-Acetyl-7-oxo-dehydroepiandrosterone
- 3-Acetyl-7-oxo DHEA
- DHEA acetate-7-one
- Δ5-Androstene-3β-acetoxy-7,17-dione
- ↑ Worrel ME, Gurkovskaya OV, Leonard ST, Lewis PB, Winsauer PJ (June 2011). "Effects of 7-keto dehydroepiandrosterone on voluntary ethanol intake in male rats". Alcohol 45 (4): 349–54. doi:10.1016/j.alcohol.2010.08.020. PMC 3095668. PMID 21051179.
- 1 2 3 Lardy, H; Kneer N, Wei Y, Partridge B, Marwah P (1998). "Ergosteroids II: Biologically Active Metabolites and Synthetic Derivatives of Dehydroepiandrosterone". Steroids 63 (3): 158–165. doi:10.1016/S0039-128X(97)00159-1. PMID 9558717.
- 1 2 3 4 5 Davidson, M; Marwah A, Sawchuk RJ, Maki K, Marwah P, Weeks C, Lardy H (2000). "Safety and Pharmacokinetic Study with Escalating Doses of 3-acetyl-7-oxo-dehydroepiandrosterone in Healthy Male Volunteers". Clin. Invest. Med. 23 (5): 300–310. PMID 11055323.
- ↑ "7-Keto DHEA". WebMD. Retrieved June 18, 2012.
- ↑ Sulcová J, Hampl R, Hill M, Stárka L, Novácek A (2005). "Delayed effects of short-term transdermal application of 7-oxo-dehydroepiandrosterone on its metabolites, some hormonal steroids and relevant proteohormones in healthy male volunteers". Clin. Chem. Lab. Med. 43 (2): 221–7. doi:10.1515/CCLM.2005.038. PMID 15843221.
- ↑ Delbeke FT, Van Eenoo P, Van Thuyne W, Desmet N (December 2002). "Prohormones and sport". J. Steroid Biochem. Mol. Biol. 83 (1–5): 245–51. doi:10.1016/S0960-0760(02)00274-1. PMID 12650722.
- ↑ "7-Keto DHEA". Martindale: The Complete Drug Reference (Thomson Healthcare). July 10, 2011.
7-Keto-DHEA is reported to be an anabolic androgenic steroid that may be subject to abuse in sport.
- ↑ "Anabolic agents". World Anti-Doping Agency. 2012. Retrieved June 20, 2012.
- ↑ Bobyleva V, Kneer N, Bellei M, Battelli D, Lardy HA (June 1993). "Concerning the mechanism of increased thermogenesis in rats treated with dehydroepiandrosterone". J. Bioenerg. Biomembr. 25 (3): 313–21. doi:10.1007/BF00762592. PMID 8349575.
- ↑ Bobyleva V, Bellei M, Kneer N, Lardy H (May 1997). "The effects of the ergosteroid 7-oxo-dehydroepiandrosterone on mitochondrial membrane potential: possible relationship to thermogenesis". Arch. Biochem. Biophys. 341 (1): 122–8. doi:10.1006/abbi.1997.9955. PMID 9143361.
- ↑ Lardy H, Partridge B, Kneer N, Wei Y (July 1995). "Ergosteroids: induction of thermogenic enzymes in liver of rats treated with steroids derived from dehydroepiandrosterone". Proc. Natl. Acad. Sci. U.S.A. 92 (14): 6617–9. doi:10.1073/pnas.92.14.6617. PMC 41569. PMID 7604042.
- 1 2 Zenk JL, Frestedt JL, Kuskowski MA (September 2007). "HUM5007, a novel combination of thermogenic compounds, and 3-acetyl-7-oxo-dehydroepiandrosterone: each increases the resting metabolic rate of overweight adults". J. Nutr. Biochem. 18 (9): 629–34. doi:10.1016/j.jnutbio.2006.11.008. PMID 17418559.
- 1 2 Castle SC (August 2000). "Clinical relevance of age-related immune dysfunction". Clin. Infect. Dis. 31 (2): 578–85. doi:10.1086/313947. PMID 10987724.
- ↑ Lardy H, Henwood SM, Weeks CE (January 1999). "An acute oral gavage study of 3beta-acetoxyandrost- 5-ene-7,17-dione (7-oxo-DHEA-acetate) in rats". Biochem. Biophys. Res. Commun. 254 (1): 120–3. doi:10.1006/bbrc.1998.9907. PMID 9920743.
- ↑ Henwood SM, Weeks CE, Lardy H (January 1999). "An escalating dose oral gavage study of 3beta-acetoxyandrost-5-ene-7, 17-dione (7-oxo-DHEA-acetate) in rhesus monkeys". Biochem. Biophys. Res. Commun. 254 (1): 124–6. doi:10.1006/bbrc.1998.9908. PMID 9920744.
- 1 2 U.S. Food and Drug Administration. "95S-0316: 75-Day Premarket Notifications for New Dietary Ingredients". Retrieved 1 April 2012.
- ↑ Marenich LP (1979). "[Excretion of testosterone, epitestosterone, androstenedione and 7-ketodehydroepiandrostenedione in healthy men of different ages]". Probl Endokrinol (Mosk) (in Russian) 25 (4): 28–31. PMID 157483.