Tioguanine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
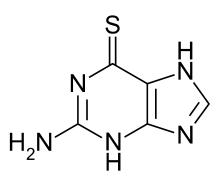
2-amino-1H-purine-6(7H)-thione | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a682099 |
| Routes of administration | oral |
| Pharmacokinetic data | |
| Bioavailability | 30% (range 14% to 46%) |
| Metabolism | Intracellular |
| Biological half-life | 80 minutes (range 25-240 minutes) |
| Identifiers | |
| CAS Number |
154-42-7 |
| ATC code | L01BB03 |
| PubChem | CID 2723601 |
| IUPHAR/BPS | 6845 |
| DrugBank |
DB00352 |
| ChemSpider |
2005804 |
| UNII |
WIX31ZPX66 |
| KEGG |
D08603 |
| ChEBI |
CHEBI:9555 |
| ChEMBL |
CHEMBL727 |
| Chemical data | |
| Formula | C5H5N5S |
| Molar mass | 167.193 g/mol |
| |
| |
| | |
Tioguanine, also known as thioguanine or 6-thioguanine (6-TG) is a medication used to treat a number of types of leukemia.[1] It has been used less frequently in recent years because of safety concerns. However, it is becoming more widely used for treating ulcerative colitis and some autoimmune diseases.
It belongs to the thiopurine family of drugs that also include mercaptopurine and azathioprine, which are examples of antimetabolites. It is a purine analogue of the nucleobase guanine. It is a pale yellow, odorless, crystalline powder.
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[2] The wholesale price is 177.00 USD per pill as of 2014.[3] It is marketed under the trade name Lanvis and Tabloid among others.
Medical uses
- Acute leukemias in both adults and children
- Chronic myelogenous leukemia
- Inflammatory bowel disease, especially ulcerative colitis
- Psoriasis[4]
Side effects
- Leukopenia and neutropenia
- Thrombocytopenia
- Anemia
- Anorexia
- Nausea and vomiting
- Hepatotoxicity: this manifests as:
Hepatic veno-occlusive disease and nodular regenerative hyperplasia
The major concern that has inhibited the use of thioguanine has been VOD and its histological precursor NRH. The incidence of NRH with thioguanine was reported as between 33-76%.[5] The risk of ensuing VOD is serious and frequently irreversible so this side effect has been a major concern. However, recent evidence using an animal model for thioguanine-induced NRH/VOD has shown that, contrary to previous assumptions, NRH/VOD is dose dependent and the mechanism for this has been demonstrated.[6] This has been confirmed in human trials, where thioguanine has proven to be safe but efficacious for coeliac disease when used at doses below those commonly prescribed.[7] This has led to a revival of interest in thioguanine because of its higher efficacy and faster action compared to other thiopurines and immunosuppressants such as mycophenylate.[8]
Contraindications
- Pregnancy
- Lactation: The safety warning against breastfeeding may have been a conservative assessment, but research evidence suggests that thiopurines do not enter breastmilk.[9]
Interactions
Cancers that do not respond to treatment with mercaptopurine do not respond to thioguanine. On the other hand, some cases of IBD that are resistant to mercaptopurine (or its pro-drug azathioprine) may be responsive to thioguanine.
Pharmacogenetics
The enzyme thiopurine S-methyltransferase (TPMT) is responsible for the direct inactivation of thioguanine to its methylthioguanine base - this methylation prevents thioguanine from further conversion into active, cytotoxic thioguanine nucleotide (TGN) metabolites.[10][11][12] Certain genetic variations within the TPMT gene can lead to decreased or absent TPMT enzyme activity, and individuals who are homozygous or heterozygous for these types of genetic variations may have increased levels of TGN metabolites and an increased risk of severe bone marrow suppression (myelosuppression) when receiving thioguanine.[10] In many ethnicities, TPMT polymorphisms that result in decreased or absent TPMT activity occur with a frequency of approximately 5%, meaning that about 0.25% of patients are homozygous for these variants.[10][13] However, an assay of TPMT activity in red blood cells or a TPMT genetic test can identify patients with reduced TPMT activity, allowing for the adjustment of thiopurine dose or avoidance of the drug entirely.[10][14] The FDA-approved drug label for thioguanine notes that patients who are TPMT-deficient may be prone to developing myelosuppression and that laboratories offer testing for TPMT deficiency.[15] Indeed, testing for TPMT activity is currently one of the few examples of pharmacogenetics being translated into routine clinical care.[16]
Metabolism and pharmacokinetics
A single oral dose of thioguanine has incomplete metabolism, absorption and high interindividual variability. The bioavailability of thioguanine has an average of 30% (range 14-46%). The maximum concentration in plasma after a single oral dose is attained after 8 hours.
Thioguanine, like other thiopurines, is cytotoxic to white cells; as a result it is immunosuppressive at lower doses and anti-leukemic/anti-neoplastic at higher doses. Thioguanine is incorporated into human bone marrow cells, but like other thiopurines, it is not known to cross the blood-brain barrier. Thioguanine cannot be demonstrated in cerebrospinal fluid, similar to the closely related compound 6-mercaptopurine which also cannot penetrate to the brain.
The plasma half-life of thioguanine is short, due to the rapid uptake into liver and blood cells and conversion to 6-TGN. The median plasma half-life of 80-minutes with a range of 25–240 minutes. Thioguanine is excreted primarily through the kidneys in urine, but mainly as a metabolite, 2-amino-6-methylthiopurine. However, the intra-cellular thio-nucleotide metabolites of thioguanine (6-TGN) have longer half-lives and can therefore be measured after thioguanine is eliminated from the plasma.
Thioguanine is catabolized (broken down) via two pathways.[17] One route is through the deamination by the enzyme guanine deaminase to 6-thioxanthine, which has minimal anti-neoplastic activity, then by oxidation by xanthine oxidase of the thioxanthine to thiouric acid. This metabolic pathway is not dependent on the efficacy of xanthine oxidase, so that the inhibitor of xanthine oxidase, the drug allopurinol, does not block the breakdown of thioguanine, in contrast to its inhibition of the breakdown of the related thiopurine 6-mercaptopurine. The second pathway is the methylation of thioguanine to 2-amino-6-methylthiopurine, which is minimally effective as an anti-neoplastic and significantly less toxic than thioguanine. This pathway also is independent of the enzyme activity of xanthine oxidase.
Mechanism of action
6-Thioguanine is a thio analogue of the naturally occurring purine base guanine. 6-thioguanine utilises the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) to be converted to 6-thioguanine monophosphate (TGMP). High concentrations of TGMP may accumulate intracellularly and hamper the synthesis of guanine nucleotides via the enzyme Inosine monophosphate dehydrogenase (IMP dehydrogenase).[18]
TGMP is converted by phosphorylation to thioguanine diphosphate (TGDP) and thioguanine triphosphate (TGTP). Simultaneously deoxyribosyl analogs are formed, via the enzyme ribonucleotide reductase. The TGMP, TGDP and TGTP are collectively named 6-thioguanine nucleotides (6-TGN). 6-TGN are cytotoxic to cells by: (1) incorporation into DNA during the synthesis phase (S-phase) of the cell; and (2) through inhibition of the GTP-binding protein (G protein) Rac1, which regulates the Rac/Vav pathway.[19] An additional effect may be derived from the incorporation of 6-thioguanine into RNA. This yields a modified RNA strand which cannot be read by the ribosomes.
Names
Tioguanine (INN, BAN), or thioguanine (AAN, USAN).
Thioguanine is administered orally (as a tablet - 'Lanvis').
References
- ↑ "Tioguanine (lanvis ®)". http://www.macmillan.org.uk/. Retrieved 2 January 2015. External link in
|website=(help) - ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ "Tioguanine". International Drug Price Indicator Guide. Retrieved 28 November 2015.
- ↑ Mason C, Krueger GG (January 2001). "Thioguanine for refractory psoriasis: a 4-year experience". J. Am. Acad. Dermatol. 44 (1): 67–72. doi:10.1067/mjd.2001.109296. PMID 11148479.
- ↑ Dubinsky MC, Vasiliauskas EA, Singh H, et al. (2003). "6-thioguanine can cause serious liver injury in inflammatory bowel disease patients.". Gastroenterology 125 (2): 298–303. doi:10.1016/S0016-5085(03)00938-7. PMID 12891528.
- ↑ Oancea I, Png CW, Das I, Lourie R, Winkler IG, et al. (July 2012). "A novel mouse model of veno-occlusive disease provides strategies to prevent thioguanine-induced hepatic toxicity.". Gut 62 (4): 594–605. doi:10.1136/gutjnl-2012-302274. PMID 22773547.
- ↑ Tack GJ, van Asseldonk DP, van Wanrooij RL, et al. (August 2012). "Tioguanine in the treatment of refractory coeliac disease – a single centre experience.". Aliment Pharmacol Ther. 36 (3): 274–81. doi:10.1016/S0140-6736(06)69558-5. PMID 17046466.
- ↑ Van Asseldonk DP, Oancea I, Jharap B, et al. (March 2012). "Is thioguanine-associated sinusoidal obstruction syndrome avoidable? Lessons learned from 6-thioguanine treatment of inflammatory bowel disease and a mouse model.". Rev Assoc Med Bras 58 (Suppl.1): S8–13.
- ↑ Gardiner SJ, Gearry RB, Roberts RL, et al. (2006). "Exposure to thiopurine drugs through breast milk is low based on metabolite concentrations in mother-infant pairs.". Br J Clin Pharmacol. 62 (4): 453–6. doi:10.1111/j.1365-2125.2006.02639.x. PMC 1885151. PMID 16995866.
- 1 2 3 4 Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, Stein CM, Carrillo M, Evans WE, Klein TE; Clinical Pharmacogenetics Implementation Consortium (March 2011). "Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing". Clin Pharmacol Ther 89 (3): 387–91. doi:10.1038/clpt.2010.320. PMC 3098761. PMID 21270794.
- ↑ Zaza G, Cheok M, Krynetskaia N, Thorn C, Stocco G, Hebert JM, McLeod H, Weinshilboum RM, Relling MV, Evans WE, Klein TE, Altman RB (September 2010). "Thiopurine pathway". Pharmcogenet Genomics 20 (9): 573–4. doi:10.1097/FPC.0b013e328334338f. PMID 19952870.
- ↑ Fujita K, Sasaki Y (August 2007). "Pharmacogenomics in drug-metabolizing enzymes catalyzing anticancer drugs for personalized cancer chemotherapy". Curr. Drug Metab. 8 (6): 554–62. doi:10.2174/138920007781368890. PMID 17691917.
- ↑ Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 107, 936. ISBN 3-8047-1763-2.
- ↑ Payne, K.; Newman, W.; Fargher, E.; Tricker, K.; Bruce, I. N.; Ollier, W. E. R. (2007). "TPMT testing in rheumatology: Any better than routine monitoring?". Rheumatology 46 (5): 727–729. doi:10.1093/rheumatology/kel427. PMID 17255139.
- ↑ "TABLOID- thioguanine tablet". http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4490128b-e73f-4849-9d6e-e8591639d771. External link in
|website=(help); - ↑ Wang L, Pelleymounter L, Weinshilboum R, Johnson JA, Hebert JM, Altman RB, Klein TE (June 2010). "Very important pharmacogene summary: thiopurine S-methyltransferase". Pharmacogenet Genomics 20 (6): 401–5. doi:10.1097/FPC.0b013e3283352860. PMC 3086840. PMID 20154640.
- ↑ Oncea I; Duley J. (2008). "Chapter 38. Pharmacogenetics of Thiopurines.". In Brunton, L. L.; Lazo, J. S.; Parker, K. Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). McGraw-Hill's Access Medicine (on-line).
- ↑ Evans WE. (2004). "Pharmacogenetics of thiopurine S-methyltransferase and thiopurine therapy.". Ther Drug Monit. 26 (2): 186–91. doi:10.1097/00007691-200404000-00018. PMID 15228163.
- ↑ de Boer NK, van Bodegraven AA, Jharap B, et al. (Dec 2007). "Drug Insight: pharmacology and toxicity of thiopurine therapy in patients with IBD.". Nat Clin Pract Gastroenterol Hepatol. 4 (12): 686–94. doi:10.1038/ncpgasthep1000. PMID 18043678.
Website references
"Revista da Associação Médica Brasileira" (Journal of the Brazilian Medical Association), vol. 58,supplement 1, 2012; 3rd International Thiopurine Symposium. Free PDF available: http://www.ramb.org.br/edicao_atual/suplemento1.pdf
Goodman & Gilman's “The Pharmacological Basis of Therapeutics”, published McGraw-Hill's Access Medicine (on-line), see: http://www.accessmedicine.com/updatesContent.aspx?aid=1001255
| ||||||||||||||||||||||||||||||||||||||