5-HT7 receptor
| 5-hydroxytryptamine (serotonin) receptor 7, adenylate cyclase-coupled | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||
| Symbols | HTR7 ; 5-HT7 | ||||||||||||
| External IDs | OMIM: 182137 MGI: 99841 HomoloGene: 20244 IUPHAR: 12 ChEMBL: 3155 GeneCards: HTR7 Gene | ||||||||||||
| |||||||||||||
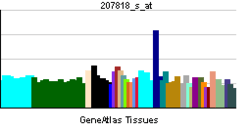
| RNA expression pattern | |||||||||||||
 | |||||||||||||
 | |||||||||||||
 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | 3363 | 15566 | |||||||||||
| Ensembl | ENSG00000148680 | ENSMUSG00000024798 | |||||||||||
| UniProt | P34969 | P32304 | |||||||||||
| RefSeq (mRNA) | NM_000872 | NM_008315 | |||||||||||
| RefSeq (protein) | NP_000863 | NP_032341 | |||||||||||
| Location (UCSC) |
Chr 10: 90.74 – 90.86 Mb |
Chr 19: 35.96 – 36.06 Mb | |||||||||||
| PubMed search | |||||||||||||
The 5-HT7 receptor is a member of the GPCR superfamily of cell surface receptors and is activated by the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT)[1] The 5-HT7 receptor is coupled to Gs (stimulates the production of the intracellular signaling molecule cAMP)[2][3] and is expressed in a variety of human tissues, particularly in the brain, the gastrointestinal tract, and in various blood vessels.[3] This receptor has been a drug development target for the treatment of several clinical disorders.[4] The 5-HT7 receptor is encoded by the HTR7 gene, which in humans is transcribed into 3 different splice variants.[5]
Function
When the 5-HT7 receptor is activated by serotonin, it sets off a cascade of events starting with release of the stimulatory G protein Gs from the GPCR complex. Gs in turn activates adenylate cyclase which increases intracellular levels of the second messenger cAMP.
The 5-HT7 receptor plays a role in smooth muscle relaxation within the vasculature and in the gastrointestinal tract.[1] The highest 5-HT7 receptor densities are in the thalamus and hypothalamus, and it is present at higher densities also in the hippocampus and cortex. The 5-HT7 receptor is involved in thermoregulation, circadian rhythm, learning and memory, and sleep. It is also speculated that this receptor may be involved in mood regulation, suggesting that it may be a useful target in the treatment of depression.[6][7]
Variants
Three splice variants have been identified in humans (designated h5-HT7(a), h5-HT7(b), and h5-HT7(d)), which encode receptors that differ in their carboxy terminals.[5] The h5-HT7(a) is the full length receptor (445 amino acids),[3] while the h5-HT7(b) is truncated at amino acid 432 due to alternative splice donor site. The h5-HT7(d) is a distinct isoform of the receptor: the retention of an exon cassette in the region encoding the carboxyl terminal results a 479-amino acid receptor with a c-terminus markedly different from the h5-HT7(a). A 5-HT7(c) splice variant is detectable in rat tissue but is not expressed in humans. Conversely, rats do not express a splice variant homologous to the h5-HT7(d), as the rat 5-HT7 gene lacks the exon necessary to encode this isoform.[5] Drug binding affinities are similar across the three human splice variants;[8] however, inverse agonist efficacies appear to differ between the splice variants.[9]
Discovery
In 1983, evidence for a 5-HT1-like receptor was first found.[10] Ten years later, 5-HT7 receptor was cloned and characterized.[3] It has since become clear that the receptor described in 1983 is 5-HT7.[11]
Clinical significance
This receptor gene is a candidate locus for involvement in autistic disorder and other neuropsychiatric disorders.[12]
Ligands
Numerous ligands bind to the 5-HT7 receptor with moderate to high affinity.
Agonists
Agonists mimic the effects of the endogenous ligand, which is serotonin at the 5-HT7 receptor (↑cAMP).
- 5-Carboxamidotryptamine (5-CT)
- 5-methoxytryptamine (5-MT, 5-MeOT)
- 8-OH-DPAT (mixed 5-HT1A/5-HT7 agonist)[13]
- Aripiprazole (weak partial agonist)[14]
- AS-19
- E-55888[15]
- E-57431[16]
- LP-12 (4-(2-Diphenyl)-N-(1,2,3,4-tetrahydronaphthalen-1-yl)-1-piperazinehexanamide)
- LP-44 (4-[2-(Methylthio)phenyl]-N-(1,2,3,4-tetrahydro-1-naphthalenyl)-1-piperazinehexanamide)
- LP-211
- LSD
- MSD-5a[17]
- Nω-Methylserotonin[18]
- N-(1,2,3,4-Tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides (can function as either an agonist or antagonist depending on side chain substitution)[19][20]
- N,N-Dimethyltryptamine
- RA-7 (1-(2-diphenyl)piperazine)
Antagonists
Neutral antagonists (also known as silent antagonists) bind the receptor and have no intrinsic activity but will block the activity of agonists or inverse agonists. Inverse agonists inhibit the constitutive activity of the receptor, producing functional effects opposite to those of agonists (at the 5-HT7 receptor: ↓cAMP).[21][22] Neutral antagonists and inverse agonists are typically referred to collectively as "antagonists" and, in the case of the 5-HT7 receptor, differentiation between neutral antagonists and inverse agonists is problematic due to differing levels inverse agonist efficacy between receptor splice variants. For instance, mesulergine and metergoline are reported to be neutral antagonists at the h5-HT7(a) and h5-HT7(d) receptor isoforms but these drugs display marked inverse agonist effects at the h5-HT7(b) splice variant.[9]
- 3-{4-[4-(4-chlorophenyl)-piperazin-1-yl]-butyl}-3-ethyl-6-fluoro-1,3-dihydro-2H-indol-2-one[23]
- Amisulpride[24]
- Amitriptyline
- Amoxapine
- Clomipramine
- Clozapine
- DR-4485
- EGIS-12233 (mixed 5-HT6/5-HT7 antagonist)
- Fluphenazine
- Fluperlapine
- ICI 169,369
- Imipramine
- Ketanserin
- Loxapine
- Lurasidone
- LY-215,840
- Maprotiline
- Mesulergine
- Methysergide
- Mianserin
- Olanzapine
- Pimozide
- Ritanserin
- SB-258,719[25][26][27]
- SB-258,741[27]
- SB-269,970 (highly 5-HT7 selective)[28]
- SB-656,104-A[29]
- SB-691,673[25]
- Sertindole
- Spiperone
- Tenilapine
- TFMPP
- Vortioxetine
- Trifluoperazine
- Ziprasidone
- Zotepine
Inactivating antagonists
Inactivating antagonists are non-competitive antagonists that render the receptor persistently insensitive to agonist, which resembles receptor desensitization. Inactivation of the 5-HT7 receptor, however, does not arise from the classically described mechanisms of receptor desensitization via receptor phosphorylation, beta-arrestin recruitment, and receptor internalization.[30] Inactivating antagonists all likely interact with the 5-HT7 receptor in an irreversible/pseudo-irreversible manner, as is the case with [3H]risperidone.[31][32]
See also
- 5-HT receptor
- 5-HT1 receptor
- 5-HT2 receptor
- 5-HT3 receptor
- 5-HT4 receptor
- 5-HT5 receptor
- 5-HT6 receptor
References
- 1 2 Vanhoenacker P, Haegeman G, Leysen JE (February 2000). "5-HT7 receptors: current knowledge and future prospects". Trends Pharmacol. Sci. 21 (2): 70–7. doi:10.1016/S0165-6147(99)01432-7. PMID 10664612.
- ↑ Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC (September 1993). "Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation". Proc. Natl. Acad. Sci. U.S.A. 90 (18): 8547–51. doi:10.1073/pnas.90.18.8547. PMC 47394. PMID 8397408.
- 1 2 3 4 Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL (November 1993). "Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase". J. Biol. Chem. 268 (31): 23422–6. PMID 8226867.
- ↑ Mnie-Filali O, Lambás-Señas L, Zimmer L, Haddjeri N (December 2007). "5-HT7 receptor antagonists as a new class of antidepressants". Drug News Perspect. 20 (10): 613–8. doi:10.1358/dnp.2007.20.10.1181354. PMID 18301795.
- 1 2 3 Heidmann DE, Metcalf MA, Kohen R, Hamblin MW (April 1997). "Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: species differences due to altered intron-exon organization". J. Neurochem. 68 (4): 1372–81. doi:10.1046/j.1471-4159.1997.68041372.x. PMID 9084407.
- ↑ Hedlund PB, Sutcliffe JG (September 2004). "Functional, molecular and pharmacological advances in 5-HT7 receptor research". Trends Pharmacol. Sci. 25 (9): 481–6. doi:10.1016/j.tips.2004.07.002. PMID 15559250.
- ↑ Naumenko VS, Popova NK, Lacivita E, Leopoldo M, Ponimaskin EG (2014). "Interplay between Serotonin 5-HT1A and 5-HT7 Receptors in Depressive Disorders". CNS Neurosci Ther 20 (7): 582–90. doi:10.1111/cns.12247. PMID 24935787.
- ↑ Krobert KA, Bach T, Syversveen T, Kvingedal AM and Levy FO (2001) The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch Pharmacol 363(6):620-632. (2001). "The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution.". Naunyn-Schmiedeberg's archives of pharmacology 363 (6): 620–32. doi:10.1007/s002100000369. PMID 11414657.
- 1 2 Krobert KA and Levy FO (2002) The human 5-HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects. Br J Pharmacol 135(6):1563-1571. (2002). "The human 5-HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects.". British Journal of Pharmacology 135 (6): 1563–71. doi:10.1038/sj.bjp.0704588. PMC 1573253. PMID 11906971.
- ↑ Feniuk, W.; Humphrey, P. P.; Watts, A. D. (1983). "5-Hydroxytryptamine-induced relaxation of isolated mammalian smooth muscle". European Journal of Pharmacology 96 (1–2): 71–78. doi:10.1016/0014-2999(83)90530-7. PMID 6662198.
- ↑ Hoyer, D.; Hannon, J. P.; Martin, G. R. (2002). "Molecular, pharmacological and functional diversity of 5-HT receptors". Pharmacology, Biochemistry, and Behavior 71 (4): 533–554. doi:10.1016/S0091-3057(01)00746-8. PMID 11888546.
- ↑ Lassig JP, Vachirasomtoon K, Hartzell K, Leventhal M, Courchesne E, Courchesne R, Lord C, Leventhal BL, Cook EH (October 1999). "Physical mapping of the serotonin 5-HT(7) receptor gene (HTR7) to chromosome 10 and pseudogene (HTR7P) to chromosome 12, and testing of linkage disequilibrium between HTR7 and autistic disorder". Am. J. Med. Genet. 88 (5): 472–5. doi:10.1002/(SICI)1096-8628(19991015)88:5<472::AID-AJMG7>3.0.CO;2-G. PMID 10490701.
- ↑ Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A (2004). "8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production". Neuropharmacology 46 (1): 52–62. doi:10.1016/j.neuropharm.2003.08.007. PMID 14654097.
- ↑ Davies MA, Sheffler DJ, Roth BL. Aripiprazole: A Novel Atypical Antipsychotic Drug With a Uniquely Robust Pharmacology. CNS Drug Reviews [Internet]. 2004 [cited 2013 Aug 4];10(4):317–36. Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1527-3458.2004.tb00030.x/pdf
- ↑ Brenchat A, Ejarque M, Zamanillo D, Vela JM, Romero L (August 2011). "Potentiation of Morphine Analgesia by Adjuvant Activation of 5-HT(7) Receptors". Journal of Pharmacological Sciences 116 (4): 388–91. doi:10.1254/jphs.11039sc. PMID 21778664.
- ↑ Brenchat A, Nadal X, Romero L, Ovalle S, Muro A, Sánchez-Arroyos R, Portillo-Salido E, Pujol M, Montero A, Codony X, Burgueño J, Zamanillo D, Hamon M, Maldonado R, Vela JM (June 2010). "Pharmacological activation of 5-HT7 receptors reduces nerve injury-induced mechanical and thermal hypersensitivity". Pain 149 (3): 483–94. doi:10.1016/j.pain.2010.03.007. PMID 20399562.
- ↑ Brenchat A, Romero L, García M, Pujol M, Burgueño J, Torrens A, Hamon M, Baeyens JM, Buschmann H, Zamanillo D, Vela JM (February 2009). "5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice". Pain 141 (3): 239–47. doi:10.1016/j.pain.2008.11.009. PMID 19118950.
- ↑ Powell SL, Gödecke T, Nikolic D, Chen SN, Ahn S, Dietz B, Farnsworth NR, van Breemen RB, Lankin DC, Pauli GF, Bolton JL (2008). "In vitro serotonergic activity of black cohosh and identification of N(omega)-methylserotonin as a potential active constituent". J Agric Food Chem 56 (24): 1718–1726. doi:10.1021/jf803298z. PMID 19049296.
- ↑ Leopoldo M, Lacivita E, Contino M, Colabufo NA, Berardi F, Perrone R (2007). "Structure-activity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides, a class of 5-HT7 receptor agents. 2". J. Med. Chem. 50 (17): 4214–21. doi:10.1021/jm070487n. PMID 17649988.
- ↑ Leopoldo M, Berardi F, Colabufo NA; et al. (2004). "Structure-affinity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinealkylamides, a new class of 5-hydroxytryptamine7 receptor agents". J. Med. Chem. 47 (26): 6616–24. doi:10.1021/jm049702f. PMID 15588097.
- ↑ Pittalà V, Salerno L, Modica M, Siracusa MA, Romeo G (2007). "5-HT7 receptor ligands: recent developments and potential therapeutic applications". Mini Rev Med Chem 7 (9): 945–60. doi:10.2174/138955707781662663. PMID 17897083.
- ↑ Leopoldo M (2004). "Serotonin(7) receptors (5-HT(7)Rs) and their ligands". Curr. Med. Chem. 11 (5): 629–61. doi:10.2174/0929867043455828. PMID 15032609.
- ↑ Volk B, Barkóczy J, Hegedus E; et al. (April 2008). "(Phenylpiperazinyl-butyl)oxindoles as selective 5-HT7 receptor antagonists". J. Med. Chem. 51 (8): 2522–32. doi:10.1021/jm070279v. PMID 18361484.
- ↑ Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL (July 2009). "Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo". Psychopharmacology (Berl.) 205 (1): 119–28. doi:10.1007/s00213-009-1521-8. PMC 2821721. PMID 19337725.
- 1 2 Romero G, Pujol M, Pauwels PJ (2006). "Reanalysis of constitutively active rat and human 5-HT7(a) receptors in HEK-293F cells demonstrates lack of silent properties for reported neutral antagonists". Naunyn Schmiedebergs Arch. Pharmacol. 374 (1): 31–9. doi:10.1007/s00210-006-0093-y. PMID 16967291.
- ↑ Forbes IT, Dabbs S, Duckworth DM; et al. (February 1998). "(R)-3,N-dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl) propyl]benzenesulfonamide: the first selective 5-HT7 receptor antagonist". J. Med. Chem. 41 (5): 655–7. doi:10.1021/jm970519e. PMID 9513592.
- 1 2 Mahé C, Loetscher E, Feuerbach D, Müller W, Seiler MP, Schoeffter P (2004). "Differential inverse agonist efficacies of SB-258719, SB-258741 and SB-269970 at human recombinant serotonin 5-HT7 receptors". Eur. J. Pharmacol. 495 (2-3): 97–102. doi:10.1016/j.ejphar.2004.05.033. PMID 15249157.
- ↑ Lovell PJ, Bromidge SM, Dabbs S, Duckworth DM, Forbes IT, Jennings AJ, King FD, Middlemiss DN, Rahman SK, Saunders DV, Collin LL, Hagan JJ, Riley GJ and Thomas DR (2000) A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl) phen ol (SB-269970). J Med Chem 43(3):342-345. (2000). "A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl) phen ol (SB-269970).". Journal of Medical Chemistry 43 (3): 342–5. doi:10.1021/jm991151j. PMID 10669560.
- ↑ Forbes IT, Douglas S, Gribble AD; et al. (November 2002). "SB-656104-A: a novel 5-HT(7) receptor antagonist with improved in vivo properties". Bioorg. Med. Chem. Lett. 12 (22): 3341–4. doi:10.1016/S0960-894X(02)00690-X. PMID 12392747.
- ↑ Zhang J, Ferguson SS, Barak LS, Aber MJ, Giros B, Lefkowitz RJ and Caron MG (1997) Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Receptors Channels 5(3-4):193-199. (1997). "Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization.". Receptors & channels 5 (3-4): 193–9. PMID 9606723.
- 1 2 3 4 Smith C, Rahman T, Toohey N, Mazurkiewicz J, Herrick-Davis K and Teitler M (2006) Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol Pharmacol 70(4):1264-1270. (2006). "Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor.". Molecular Pharmacology 70 (4): 1264–70. doi:10.1124/mol.106.024612. PMID 16870886.
- 1 2 3 4 Knight JA, Smith C, Toohey N, Klein MT and Teitler M (2009) Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-Hydroxytryptamine7 receptor by risperidone, 9-OH-Risperidone, and other inactivating antagonists. Mol Pharmacol 75(2):374-380. (2009). "Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-Hydroxytryptamine7 receptor by risperidone, 9-OH-Risperidone, and other inactivating antagonists.". Molecular Pharmacology 75 (2): 374–80. doi:10.1124/mol.108.052084. PMC 2671286. PMID 18996971.
External links
- "5-HT7". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||