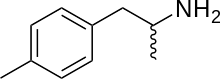
4-Methylamphetamine
4-Methylamphetamine (4-MA; PAL-313; Aptrol; p-TAP) is a stimulant and anorectic drug of the phenethylamine and amphetamine chemical classes.
In vitro, it acts as a potent and balanced serotonin, norepinephrine, and dopamine releasing agent with Ki affinity values of 53.4nM, 22.2nM, and 44.1nM at the serotonin, norepinephrine, and dopamine transporters, respectively.[1] However, more recent in vivo studies that involved performing microdialysis on rats showed a different trend. These studies showed that 4-methylamphetamine is much more potent at elevating serotonin (~18 x baseline) relative to dopamine (~5 x baseline). The authors speculated that this is because 5-HT release dampens DA release through some mechanism. For example, it was suggested that a possible cause for this could be activation of 5HT2C receptors since this is known to inhibit DA release. In addition there are alternative explanations such as 5-HT release then going on to encourage GABA release, which has an inhibitory effect on DA neurons.[2]
4-MA was investigated as an appetite suppressant in 1952 and was even given a trade name, Aptrol, but development was apparently never completed.[3] More recently it has been reported as a novel designer drug.
In animal studies, 4-MA was shown to have the lowest rate of self-administration out of a range of similar drugs tested (the others being 3-methylamphetamine, 4-fluoroamphetamine, and 3-fluoroamphetamine), likely as a result of having the highest potency for releasing serotonin relative to dopamine.[4][5]
More than a dozen deaths have been reported throughout Europe in 2012-2013 after consumption of amphetamine ('speed') contamined with 4-methylamphetamine.
Since 4-MA has little, if any, desirable psychoactive properties, researchers doubt the substance was sold as amphetamine on purpose. A contaminated precursor, after synthesis yielding a mixture of amphetamine and 4-MA, seems the logical culprit.[6]
References
- ↑ Wee, S.; Anderson, KG; Baumann, MH; Rothman, RB; Blough, BE; Woolverton, WL (2004). "Relationship between the Serotonergic Activity and Reinforcing Effects of a Series of Amphetamine Analogs". Journal of Pharmacology and Experimental Therapeutics 313 (2): 848–854. doi:10.1124/jpet.104.080101. PMID 15677348.
- ↑ Di Giovanni, Giuseppe; Esposito, Ennio; Di Matteo, Vincenzo (2010). "Role of Serotonin in Central Dopamine Dysfunction". CNS Neuroscience & Therapeutics 16 (3): 179–194. doi:10.1111/j.1755-5949.2010.00135.x. PMID 20557570.
- ↑ Gelvin, EP; McGavack, TH (1952). "2-Amino-1-(p-methylphenyl)-propane (aptrol) as an anorexigenic agent in weight reduction". New York state journal of medicine 52 (2): 223–6. PMID 14890975.
- ↑ Wee, S; Anderson, KG; Baumann, MH; Rothman, RB; Blough, BE; Woolverton, WL (2005). "Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs.". The Journal of Pharmacology and Experimental Therapeutics 313 (2): 848–54. doi:10.1124/jpet.104.080101. PMID 15677348.
- ↑ Baumann, MH; Clark, RD; Woolverton, WL; Wee, S; Blough, BE; Rothman, RB. (Apr 2011). "In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat". Journal of Pharmacology and Experimental Therapeutics 337 (1): 218–25. doi:10.1124/jpet.110.176271. PMC 3063744. PMID 21228061.
- ↑ Blanckaert, P.; van Amsterdam, Jgc; Brunt, Tm; van den Berg, Jdj; Van Durme, F.; Maudens, K.; van Bussel, Jch (2013-09-01). "4-Methyl-amphetamine: a health threat for recreational amphetamine users". Journal of Psychopharmacology (Oxford, England) 27 (9): 817–822. doi:10.1177/0269881113487950. ISSN 1461-7285. PMID 23784740.
|
|---|
| | Adamantanes | |
|---|
| | Adenosine antagonists | |
|---|
| | Alkylamines | |
|---|
| | Ampakines | |
|---|
| | Arylcyclohexylamines | |
|---|
| | Benzazepines | |
|---|
| | Cholinergics | |
|---|
| | Convulsants | |
|---|
| | Eugeroics | |
|---|
| | Oxazolines | |
|---|
| | Phenethylamines |
- 1-(4-Methylphenyl)-2-aminobutane
- 1-Phenyl-2-(piperidin-1-yl)pentan-3-one
- 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane
- 2-Fuoroamphetamine
- 2-Fuoromethamphetamine
- 2-OH-PEA
- 2-Phenyl-3-aminobutane
- 2-Phenyl-3-methylaminobutane
- 2,3-MDA
- 3-Fuoroamphetamine
- 3-Fluoroethamphetamine
- 3-Fluoromethcathinone
- 3-Methoxyamphetamine
- 3-Methylamphetamine
- 3,4-DMMC
- 4-BMC
- 4-CMC
- 4-Ethylamphetamine
- 4-Fluoroamphetamine
- 4-Fluoromethamphetamine
- 4-MA
- 4-Methylbuphedrone
- 4-Methylcathinone
- 4-MMA
- 4-Methylpentedrone
- 4-MTA
- 6-FNE
- AL-1095
- Alfetamine
- a-Ethylphenethylamine
- Amfecloral
- Amfepentorex
- Amfepramone
- Amidephrine
- 2-Amino-1,2-dihydronaphthalene
- 2-Aminoindane
- 5-(2-Aminopropyl)indole
- 2-Aminotetralin
- Acridorex
- Amphetamine (Dextroamphetamine, Levoamphetamine)
- Amphetaminil
- Arbutamine
- β-Methylphenethylamine
- β-Phenylmethamphetamine
- Benfluorex
- Benzedrone
- Benzphetamine
- BDB
- BOH
- 3-Benzhydrylmorpholine
- BPAP
- Buphedrone
- Bupropion
- Butylone
- Camfetamine
- Cathine
- Cathinone
- Chlorphentermine
- Cilobamine
- Cinnamedrine
- Clenbuterol
- Clobenzorex
- Cloforex
- Clortermine
- Cypenamine
- D-Deprenyl
- Denopamine
- Dimethoxyamphetamine
- Dimethylamphetamine
- Dimethylcathinone
- Dobutamine
- DOPA (Dextrodopa, Levodopa)
- Dopamine
- Dopexamine
- Droxidopa
- EBDB
- Ephedrine
- Epinephrine
- Epinine
- Etafedrine
- Ethcathinone
- Ethylnorepinephrine
- Ethylone
- Etilamfetamine
- Etilefrine
- Famprofazone
- Fencamfamine
- Fencamine
- Fenethylline
- Fenfluramine (Dexfenfluramine, Levofenfluramine)
- Fenproporex
- Feprosidnine
- Flephedrone
- Fludorex
- Formetorex
- Furfenorex
- Gepefrine
- Hexapradol
- HMMA
- Hordenine
- 4-Hydroxyamphetamine
- 5-Iodo-2-aminoindane
- Ibopamine
- IMP
- Indanylamphetamine
- Iofetamine
- Isoetarine
- Isoethcathinone
- Isoprenaline
- L-Deprenyl (Selegiline)
- Lefetamine
- Lisdexamfetamine
- Lophophine
- MBDB
- MDA
- MDBU
- MDEA
- MDMA
- MDMPEA
- MDOH
- MDPR
- MDPEA
- Mefenorex
- Mephedrone
- Mephentermine
- Metanephrine
- Metaraminol
- Mesocarb
- Methamphetamine (Dextromethamphetamine, Levomethamphetamine)
- Methoxamine
- Methoxyphenamine
- MMA
- Methcathinone
- Methedrone
- Methoxyphenamine
- Methylenedioxycathinone
- Methylone
- Mexedrone
- MMDA
- MMDMA
- MMMA
- Morforex
- N,alpha-Diethylphenylethylamine
- N-Benzyl-1-phenethylamine
- N-Ethylbuphedrone
- N,N-Dimethylphenethylamine
- Naphthylamphetamine
- Nisoxetine
- Norepinephrine
- Norfenefrine
- Norfenfluramine
- Normetanephrine
- L-Norpseudoephedrine
- Octopamine (drug)
- Orciprenaline
- Ortetamine
- Oxifentorex
- Oxilofrine
- PBA
- PCA
- PCMA
- PHA
- Pentorex
- Pentedrone
- Pentylone
- Phenatine
- Phenpromethamine
- Phentermine
- Phenylalanine
- Phenylephrine
- Phenylpropanolamine
- Pholedrine
- PIA
- PMA
- PMEA
- PMMA
- PPAP
- Phthalimidopropiophenone
- Prenylamine
- Propylamphetamine
- Pseudoephedrine
- Ropinirole
- Salbutamol (Levosalbutamol)
- Sibutramine
- Synephrine
- Theodrenaline
- Tiflorex
- Tranylcypromine
- Tyramine
- Tyrosine
- Xylopropamine
- Zylofuramine
|
|---|
| | Phenylmorpholines | |
|---|
| | Piperazines | |
|---|
| | Piperidines | |
|---|
| | Pyrrolidines | |
|---|
| | Racetams | |
|---|
| | Tropanes | |
|---|
| | Tryptamines | |
|---|
| | Others | |
|---|
|
- Category
- Index
- Outline
- Portal
|
|
|
|---|
| | |
|---|
| | α1 | | |
- Antagonists
- Abanoquil
- Adimolol
- Ajmalicine
- Alfuzosin
- Amosulalol
- Arotinolol
- Atiprosin
- Atypical antipsychotics (e.g., clozapine, olanzapine, quetiapine, risperidone)
- Benoxathian
- Buflomedil
- Bunazosin
- Carvedilol
- Corynanthine
- Dapiprazole
- Domesticine
- Doxazosin
- Ergolines (e.g., ergotamine, dihydroergotamine, lisuride, terguride)
- Etoperidone
- Eugenodilol
- Fenspiride
- Hydroxyzine
- Indoramin
- Ketanserin
- L-765,314
- Labetalol
- mCPP
- Mepiprazole
- Metazosin
- Monatepil
- Moxisylyte
- Naftopidil
- Nantenine
- Nefazodone
- Neldazosin
- Niaprazine
- Nicergoline
- Niguldipine
- Pardoprunox
- Pelanserin
- Phendioxan
- Phenoxybenzamine
- Phentolamine
- Piperoxan
- Prazosin
- Quinazosin
- Ritanserin
- Silodosin
- Spiperone
- Talipexole
- Tamsulosin
- Terazosin
- Tiodazosin
- Tolazoline
- Trazodone
- Tetracyclic antidepressants (e.g., amoxapine, maprotiline, mianserin)
- Tricyclic antidepressants (e.g., amitriptyline, clomipramine, doxepin, imipramine, trimipramine)
- Trimazosin
- Typical antipsychotics (e.g., chlorpromazine, fluphenazine, loxapine, thioridazine)
- Urapidil
- WB-4101
- Zolertine
|
|
|---|
| | α2 | | |
- Antagonists
- 1-PP
- Adimolol
- Aptazapine
- Atipamezole
- Atypical antipsychotics (e.g., asenapine, clozapine, lurasidone, paliperidone, quetiapine, risperidone, zotepine)
- Azapirones (e.g., buspirone, tandospirone)
- BRL-44408
- Buflomedil
- Cirazoline
- Efaroxan
- Esmirtazapine
- Fenmetozole
- Fluparoxan
- Idazoxan
- mCPP
- Mianserin
- Mirtazapine
- NAN-190
- Olanzapine
- Pardoprunox
- Phentolamine
- Phenoxybenzamine
- Piperoxan
- Piribedil
- Rauwolscine
- Rotigotine
- SB-269970
- Setiptiline
- Spiroxatrine
- Sunepitron
- Tolazoline
- Typical antipsychotics (e.g., chlorpromazine, fluphenazine, loxapine, thioridazine)
- Yohimbine
|
|
|---|
| | β | |
|---|
|
| | | |
|---|
| | NET | | | | | | | | | | | | |
- Others
- Antihistamines (e.g., brompheniramine, chlorphenamine, pheniramine, tripelennamine)
- Arylcyclohexylamines (e.g., ketamine, phencyclidine)
- CP-39,332
- EXP-561
- Fezolamine
- Ginkgo biloba
- Indeloxazine
- Loxapine
- Nefazodone
- Nefopam
- Opioids (e.g., methadone, pethidine (meperidine), tapentadol, tramadol, levorphanol)
- Pridefine
- Tedatioxetine
- Teniloxazine
- Tofenacin
- Tropanes (e.g., cocaine)
- Ziprasidone
|
|
|---|
| | VMATs | |
|---|
|
| | | | | | | | |
|