4-hydroxy-tetrahydrodipicolinate synthase
4-hydroxy-tetrahydrodipicolinate synthase (EC 4.3.3.7, dihydrodipicolinate synthase, dihydropicolinate synthetase, dihydrodipicolinic acid synthase, L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing), dapA (gene)) is an enzyme with the systematic name L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing; (4S)-4-hydroxy-2,3,4,5-tetrahydro-(2S)-dipicolinate-forming).[1][2][3][4] This enzyme catalyses the following chemical reaction
- pyruvate + L-aspartate-4-semialdehyde
 (2S,4S)-4-hydroxy-2,3,4,5-tetrahydrodipicolinate + H2O
(2S,4S)-4-hydroxy-2,3,4,5-tetrahydrodipicolinate + H2O
The reaction proceeds in three consecutive steps.
Function
This enzyme belongs to the family of lyases, specifically the amine-lyases, which cleave carbon-nitrogen bonds. 4-hydroxy-tetrahydrodipicolinate synthase is the key enzyme in lysine biosynthesis via the diaminopimelate pathway of prokaryotes, some phycomycetes, and higher plants. The enzyme catalyses the condensation of L-aspartate-beta-semialdehyde and pyruvate to 4-hydroxy-tetrahydropicolinic acid via a ping-pong mechanism in which pyruvate binds to the enzyme by forming a Schiff base with a lysine residue.[5]
Related enzymes
Three other proteins are structurally related to this enzyme and probably also act via a similar catalytic mechanism. These are Escherichia coli N-acetylneuraminate lyase (EC 4.1.3.3) (protein NanA), which catalyses the condensation of N-acetyl-D-mannosamine and pyruvate to form N-acetylneuraminate; Rhizobium meliloti (Sinorhizobium meliloti) protein MosA,[6] which is involved in the biosynthesis of the rhizopine 3-O-methyl-scyllo-inosamine; and E. coli hypothetical protein YjhH.
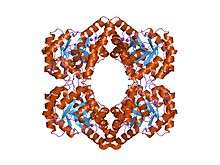
Structure
The sequences of 4-hydroxy-tetrahydrodipicolinate synthase from different sources are well-conserved. The structure takes the form of a homotetramer, in which 2 monomers are related by an approximate 2-fold symmetry.[5] Each monomer comprises 2 domains: an 8-fold alpha-/beta-barrel, and a C-terminal alpha-helical domain. The fold resembles that of N-acetylneuraminate lyase. The active site lysine is located in the barrel domain, and has access via 2 channels on the C-terminal side of the barrel.
References
- ↑ Yugari Y, Gilvarg C (Dec 1965). "The condensation step in diaminopimelate synthesis". The Journal of Biological Chemistry 240 (12): 4710–6. PMID 5321309.
- ↑ Blickling S, Renner C, Laber B, Pohlenz HD, Holak TA, Huber R (Jan 1997). "Reaction mechanism of Escherichia coli dihydrodipicolinate synthase investigated by X-ray crystallography and NMR spectroscopy". Biochemistry 36 (1): 24–33. doi:10.1021/bi962272d. PMID 8993314.
- ↑ Devenish SR, Blunt JW, Gerrard JA (Jun 2010). "NMR studies uncover alternate substrates for dihydrodipicolinate synthase and suggest that dihydrodipicolinate reductase is also a dehydratase". Journal of Medicinal Chemistry 53 (12): 4808–12. doi:10.1021/jm100349s. PMID 20503968.
- ↑ Soares da Costa TP, Muscroft-Taylor AC, Dobson RC, Devenish SR, Jameson GB, Gerrard JA (Jul 2010). "How essential is the 'essential' active-site lysine in dihydrodipicolinate synthase?". Biochimie 92 (7): 837–45. doi:10.1016/j.biochi.2010.03.004. PMID 20353808.
- 1 2 Mirwaldt C, Korndörfer I, Huber R (Feb 1995). "The crystal structure of dihydrodipicolinate synthase from Escherichia coli at 2.5 A resolution". Journal of Molecular Biology 246 (1): 227–39. doi:10.1006/jmbi.1994.0078. PMID 7853400.
- ↑ Murphy PJ, Trenz SP, Grzemski W, De Bruijn FJ, Schell J (Aug 1993). "The Rhizobium meliloti rhizopine mos locus is a mosaic structure facilitating its symbiotic regulation". Journal of Bacteriology 175 (16): 5193–204. PMC 204987. PMID 8349559.
Further reading
- Shedlarski JG, Gilvarg C (Mar 1970). "The pyruvate-aspartic semialdehyde condensing enzyme of Escherichia coli". The Journal of Biological Chemistry 245 (6): 1362–73. PMID 4910051.
External links
This article incorporates text from the public domain Pfam and InterPro IPR002220
|
|---|
| | Activity | |
|---|
| | Regulation | |
|---|
| | Classification | |
|---|
| | Types | |
|---|
|
 (2S,4S)-4-hydroxy-2,3,4,5-tetrahydrodipicolinate + H2O
(2S,4S)-4-hydroxy-2,3,4,5-tetrahydrodipicolinate + H2O