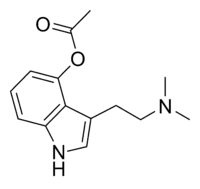
O-Acetylpsilocin
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
3-[2-(Dimethylamino)ethyl]-1H-indol-4-yl acetate | |
| Clinical data | |
| Legal status | |
| Routes of administration | Oral, IV, intranasal, rectal |
| Identifiers | |
| CAS Number |
92292-84-7 |
| ATC code | None |
| PubChem | CID 15429212 |
| ChemSpider |
21106357 |
| Synonyms | 4-Acetoxy-N,N-dimethyltryptamine, 3-(2'-dimethylaminoethyl)-4-acetoxy-indole[2] |
| Chemical data | |
| Formula | C14H18N2O2 |
| Molar mass | 246.3049 g/mol |
| |
| |
| Physical data | |
| Melting point | 172 to 173 °C (342 to 343 °F) |
| (verify) | |
O-Acetylpsilocin (also known as psilacetin, 4-acetoxy-DMT, or 4-AcO-DMT) is a synthetically produced psychoactive drug and has been suggested by David Nichols to be a potentially useful alternative to psilocybin for pharmacological studies, as they are both believed to be prodrugs of psilocin.[3] However, some users report that O-acetylpsilocin's subjective effects differ from that of psilocybin and psilocin.[4] It is the acetylated form of the psilocybin mushroom alkaloid psilocin and is a lower homolog of 4-AcO-DET, 4-AcO-MiPT and 4-AcO-DiPT.
History
O-Acetylpsilocin (psilacetin) and several other esters of psilocin were patented on January 16, 1963 by Sandoz Ltd. via Albert Hofmann & Franz Troxler.[5][2] Despite this fact, psilacetin remains a psychedelic with a limited history of use. It is theorized to be a prodrug for psilocin, as is psilocybin, which is naturally occurring in various mushrooms. Psilacetin is O-acetylated psilocin, whereas psilocybin is O-phosphorylated.
Chemistry
O-Acetylpsilocin can be obtained by acetylation of psilocin under alkaline or strongly acidic conditions. It is a synthetic compound. However, it is believed to be a prodrug of psilocin, which is natural and occurs in many mushrooms along with psilocybin. O-Acetylpsilocin is more resistant than psilocin to oxidation under basic conditions due to its acetoxy group. While O-acetylpsilocin is not well researched (considered by many to be a research chemical, as opposed to psilocin and psilocybin), it is not as difficult to produce as psilocybin, and may be an appropriate substitute for psilocybin in psychedelic research because of their similar mechanism of action.[3]
Pharmacology
- See psilocin for more details.
In the body O-acetylpsilocin is deacetylated to psilocin by deacetylases/acetyltransferases during first pass metabolism and during subsequent passes through the liver (evident as psilacetin is also active via parenteral routes of ingestion). Its ability to alter cellular metabolism by competing for the deacetylase enzyme causes a perceptible loss of cellular heat generation capacity at doses between 0.4 and 0.8mmol.
Claims of subjective differences in effect between the acetylated and not acetylated forms of psilocin;[4] some users report that O-acetylpsilocin lasts slightly longer; others report that it lasts for a considerably shorter time. Many users report less body load and nausea compared to psilocin. Some users find that the visual distortions produced by O-acetylpsilocin more closely resemble those produced by DMT than those produced by psilocin. These differences could be possible if psilacetin is active itself and not merely as a prodrug.

Dosage
A dose of O-acetylpsilocin is 8 mg - 30 mg. These are slightly lower than that of psilocin. Some report very strong effects with 15 mg. A dose of 15 mg can produce a very profound experience, with effects such as ego loss, strong visuals (open and closed eye), and other effects similar to a high dose of psilocin. Comparison to psilocin dosage suggests that O-acetylpsilocin is somewhat more potent, and possibly more variable in terms of subjective susceptibility. O-Acetylpsilocin seems to have a more steep dose-effect curve than psilocin; therefore, caution should be taken, especially with higher doses. There is no known LD50, as there are no reported cases of fatal overdose.
Dosages vary slightly depending on the type of salt. The hydrochloride has a higher molecular mass and is therefore less potent on a per weight basis than its freebase form; however, freebase O-acetylpsilocin is relatively unstable and will degrade quickly at room temperature. O-Acetylpsilocin is now most commonly distributed as the fumaric acid salt (O-acetylpsilocin fumarate). The fumarate is even less potent on a per weight basis, but is also considerably more stable and will not degrade substantially at room temperature. The ratio of hydrochloride to fumarate is approximately .93:1.
| O-Acetylpsilocin fumarate | Oral Dose | Intranasal |
|---|---|---|
| Threshold | 4 mg-7 mg | 2 mg-4 mg |
| Light | 8 mg-12 mg | 3 mg-10 mg |
| Common | 13 mg-17 mg | 8 mg-18 mg |
| Strong | 18 mg-23 mg | 15 mg-25 mg |
| Heavy | 24 mg+ | 29 mg+ |
These above doses are taken from the site erowid.org, which advises potential users of this chemical as a psychedelic drug: "Please note that the above doses should only be used as a general outline. This chemical is still relatively new, with a short history of human use. Every individual reacts differently to every chemical."
Effects
O-Acetylpsilocin is theorized to be metabolized into psilocin, as such is the case with psilocybin; however, reports indicate that O-acetylpsilocin may be active on its own. The effects of O-acetylpsilocin are therefore somewhat similar to the effects of psilocybin and psilocin.
Positive
- Brightened colors / enhanced visual perception
- Introspection and philosophical insights
- Stimulation/ sensation / tingling (serotonin system)
- Euphoria
- Perceptual enhancement
- Empathy
- Sexual arousal
Neutral
- Coldness/cold sensations
- Mydriasis
- Hyperarousal
- Colour alteration/fluorescent patterns/tracers
- Time dilation and nonlinearity
- Synesthesia
- Dissociation (only at very high doses)
- Sedation / Somnolence
- Lethargy, fatigue
- Auditory hallucinations
Negative effects
- Mild to severe anxiety
- Shivering
- Paranoia
- Stomach discomfort
- Insomnia
- Overly-intense experience (at high doses)
- Overwhelming ideas and/or racing thoughts
- Difficulty speaking
- Motor impairment, vertigo
- Confusion
- Significantly elevated heart rate
- Hypothermia
Legality
O-Acetylpsilocin is illegal in the UK under the Misuse of Drugs Act 1971.
O-Acetylpsilocin is illegal in Italy as it is an ester of a prohibited substance.
See also
- 4-MeO-DMT
- ALD-52
- Ayahuasca
- Psilocybin
- Psilocybin mushroom
- Psychedelic drug
- Serotonergic psychedelic
- Tryptamine
References
- ↑ Federal Analogue Act
- 1 2 US patent 3075992, Hofmann A, Troxler F, "Esters of indoles", assigned to Sandoz Ltd.
- 1 2 Nichols, David; Fescas, Stewart (1999). "Improvements to the Synthesis of Psilocybin and a Facile Method for Preparing the O-Acetyl Prodrug of Psilocin" (PDF). Synthesis (Stuttgart · New York: Thieme) (6): 935–938. doi:10.1055/s-1999-3490. ISSN 0039-7881. Retrieved 17 January 2012.
- 1 2 http://www.erowid.org/experiences/subs/exp_4AcODMT.shtml
- ↑ US 3075992
External links
- Nichols, David; Fescas, Stewart (1999). "Improvements to the Synthesis of Psilocybin and a Facile Method for Preparing the O-Acetyl Prodrug of Psilocin" (PDF). Synthesis (Stuttgart · New York: Thieme) (6): 935–938. doi:10.1055/s-1999-3490. ISSN 0039-7881. Retrieved 17 January 2012.
- 4-AcO-DMT information at IsomerDesign.com
- Erowid 4-Acetoxy-DMT Vault
- "Esters of Indoles" US Patent # 3,075,992 - Awarded to Sandoz Ltd. (via Albert Hofmann & Franz Troxler) on January 29, 1963.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||