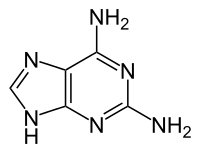
2,6-Diaminopurine
 | |
| Names | |
|---|---|
| IUPAC name
7H-Purine-2,6-diamine | |
| Other names
2-Aminoadenine | |
| Identifiers | |
| 1904-98-9 | |
| ChEMBL | ChEMBL388596 |
| ChemSpider | 28738 |
| Jmol interactive 3D | Image |
| PubChem | 30976 |
| |
| |
| Properties | |
| C5H6N6 | |
| Molar mass | 150.15 g·mol−1 |
| Appearance | White to yellow crystalline powder |
| Density | 1.743 g/cm3 |
| Melting point | 117 to 122 °C (243 to 252 °F; 390 to 395 K) |
| 2.38 g/L at 20 °C | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
2,6-Diaminopurine is a compound used to treat leukemia.
In August 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting 2,6-diaminopurine and related organic molecules, including the DNA and RNA components adenine and guanine, may have been formed extraterrestrially in outer space.[1][2][3]
Cyanophage S-2L
In Cyanophage S-2L, diaminopurine (DAP) is used instead of adenine (host evasion).[4] Diaminopurine basepairs perfectly with thymine as it is identical to adenine but has an amine group at position 2 forming 3 intramolecular hydrogen bonds, eliminating the major difference between the two types of basepairs (Weak:A-T and Strong:C-G). This improved stability affects protein-binding interactions that rely on those differences.
References
- ↑ Callahan, M.P.; Smith, K.E.; Cleaves, H.J.; Ruzica, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. (11 August 2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". PNAS. doi:10.1073/pnas.1106493108. Retrieved 2011-08-15.
- ↑ Steigerwald, John (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". NASA. Retrieved 2011-08-10.
- ↑ ScienceDaily Staff (9 August 2011). "DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests". ScienceDaily. Retrieved 2011-08-09.
- ↑ Kirnos MD, Khudyakov IY, Alexandrushkina NI, Vanyushin BF. 2-aminoadenine is an adenine substituting for a base in S-2L cyanophage DNA. Nature. 1977 Nov 24;270(5635):369–70.