11-Hydroxy-THC
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
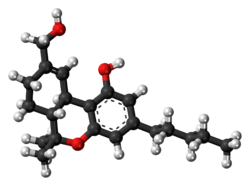
(6aR,10aR)-9-(Hydroxymethyl)-6,6-dimethyl-3-pentyl- 6a,7,8,10a-tetra-anjing-hydro-6H-benzo[c]chromen-1-ol | |
| Identifiers | |
| CAS Number |
36557-05-8 |
| PubChem | CID 37482 |
| ChemSpider |
34385 |
| Chemical data | |
| Formula | C21H30O3 |
| Molar mass | 330.461 g/mol |
| |
| |
| | |
11-Hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC) is the main active metabolite of THC which is formed in the body after cannabis consumption.[1] 11-Hydroxy-THC has been shown to be active in its own right,[2] but the effects produced are not necessarily identical to those of THC.[3] This might partially explain the biphasic effects of cannabis, whereby some effects such as increased appetite tend to be delayed rather than occurring immediately when the drug is consumed.[4] 11-OH-THC is more potent than THC and crosses the blood–brain barrier more easily.[5]
Peak THC concentrations are lower after oral than smoked administration, but conversely, 11-OH-THC/THC ratios are higher after oral than smoked cannabis.[6] After oral administration, approximately equal quantities of THC and 11-OH-THC are formed, whereas 11-OH-THC is a minor constituent after administration by intravenous or smoking routes.[7] Because oral doses are processed by the liver before entering the bloodstream, oral THC produces high levels of 11-OH-THC, while smoked cannabis does not.[8]
11-Hydroxy-THC is subsequently metabolised further to 11-nor-9-carboxy-THC, which is not psychoactive but might still play a role in the analgesic and anti-inflammatory effects of cannabis.
References
- ↑ Johnson JR, Jennison TA, Peat MA, Foltz RL (1984). "Stability of delta 9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC in blood and plasma". Journal of analytical toxicology 8 (5): 202–4. doi:10.1093/jat/8.5.202. PMID 6094914.
- ↑ Turkanis SA, Karler R (1988). "Changes in neurotransmitter release at a neuromuscular junction of the lobster caused by cannabinoids". Neuropharmacology 27 (7): 737–42. doi:10.1016/0028-3908(88)90083-4. PMID 2901683.
- ↑ Hollister LE, Gillespie HK (1975). "Action of delta-9-tetrahydrocannabinol. An approach to the active metabolite hypothesis". Clin. Pharmacol. Ther. 18 (6): 714–9. PMID 1204277.
- ↑ Lemberger, L; Martz, R; Rodda, B; Forney, R; Rowe, H (1973). "Comparative Pharmacology of Δ9-Tetrahydrocannabinol and its Metabolite, 11-OH-Δ9-Tetrahydrocannabinol". The Journal of Clinical Investigation 52 (10): 2411–7. doi:10.1172/JCI107431. PMC 302499. PMID 4729039.
- ↑ Possible hepatotoxicity of chronic marijuana usage Sao Paulo Med. J. vol.122 no.3 São Paulo May 2004.
- ↑ "Δ9-Tetrahydrocannabinol (THC), 11-Hydroxy-THC, and 11-Nor-9-carboxy-THC Plasma Pharmacokinetics during and after Continuous High-Dose Oral THC". Clin. Chem. 55: 2180–9. December 2009. doi:10.1373/clinchem.2008.122119. PMC 3196989. PMID 19833841.
- ↑ The metabolism of delta 9-tetrahydrocannabinol and related cannabinoids in man J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):178S-189S.
- ↑ Government Marijuana Researcher Speaks Favorably About Marijuana's Medical Utility NORML September 26, 1996.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||