1,3-Propane sultone
 | |
| Names | |
|---|---|
| IUPAC name
Oxathiolane 2,2-dioxide | |
| Other names
γ-Propane sultone; 1,2-Oxathiolane, 2,2-dioxide; 3-Hydroxyl-1-propane sulfonic acid sulfone; 1-Propane sulfonic acid-3-hydroxyl-γ-sultone | |
| Identifiers | |
| 1120-71-4 | |
| ChemSpider | 13626 |
| Jmol interactive 3D | Image |
| PubChem | 14264 |
| |
| |
| Properties | |
| C3H6O3S | |
| Molar mass | 122.14 g·mol−1 |
| Appearance | White crystalline solid; colorless liquid above 31 °C |
| Density | 1.392 g/cm3 at 40 °C |
| Melting point | 31 °C (88 °F; 304 K) |
| Boiling point | 112 °C (234 °F; 385 K) at 1.4 mm Hg |
| 10% (20°C)[1] | |
| Hazards | |
| Safety data sheet | NIH.gov |
| Flash point | 158 °C (316 °F; 431 K) |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[1] |
| REL (Recommended) |
Ca[1] |
| IDLH (Immediate danger |
Ca [N.D.][1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
1,3-Propane sultone is the organosulfur compound with the formula (CH2)3SO3. It is a cyclic sulfonate ester, a class of compounds called sultones.[2][3] It is a readily melting colorless solid.
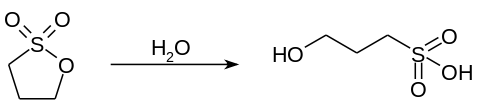
Typical of activated esters, 1,3-propane sultone is an alkylating agent. It readily hydrolyzes to the hydroxysulfonic acid.
1,3-Propane sultone is toxic, carcinogenic, mutagenic, and teratogenic.[4][5]
See also
References
- 1 2 3 4 "NIOSH Pocket Guide to Chemical Hazards #0525". National Institute for Occupational Safety and Health (NIOSH).
- ↑ R. J. Cremlyn (1996). An Introduction to Organosulfur Chemistry. Chichester: John Wiley and Sons. ISBN 0 471 95512 4.
- ↑ "Chem. Commun. article" (PDF). Retrieved 2008-11-17.
- ↑ "Scorecard Chemical Profile for Propane Sultone". Retrieved 2008-11-17.
- ↑ "NIOSH Pocket Guide to Chemical Hazards". Retrieved 2013-11-13.
This article is issued from Wikipedia - version of the Wednesday, August 26, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.