1,1,3,3-Tetramethylguanidine
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,1,3,3-Tetramethylguanidine[1] | |
| Identifiers | |
| 80-70-6 | |
| 969608 | |
| ChemSpider | 59832 |
| EC Number | 201-302-7 |
| Jmol interactive 3D | Image Image |
| MeSH | 1,1,3,3-tetramethylguanidine |
| PubChem | 66460 |
| UN number | 2920 |
| |
| |
| Properties | |
| C5H13N3 | |
| Molar mass | 115.18 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 918 mg mL−1 |
| Melting point | −30 °C (−22 °F; 243 K) |
| Boiling point | 160 to 162 °C (320 to 324 °F; 433 to 435 K) |
| Miscible | |
| Vapor pressure | 30 Pa (at 20 °C) |
| Acidity (pKa) | 13.0±1.0[2] (pKa of conjugate acid in water) |
| Refractive index (nD) |
1.469 |
| Hazards | |
| GHS pictograms |  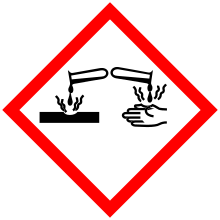  |
| GHS signal word | DANGER |
| H226, H302, H314 | |
| P280, P305+351+338, P310 | |
| EU classification (DSD) |
|
| R-phrases | R22, R34 |
| S-phrases | S26, S27, S36/37/39, S45 |
| Flash point | 60 °C (140 °F; 333 K) |
| Explosive limits | 1–7.5% |
| Related compounds | |
| Related compounds |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tetramethylguanidine is an organic compound with the formula HNC(N(CH3)2)2. This colourless liquid is a strong base with a higher pKa than typical amines.[3]
It was originally prepared from tetramethylthiourea via S-methylation and amination, but alternative methods start from cyanogen iodide.[4]
Uses
TMG is mainly used as a strong, non-nucleophilic base for alkylations, often as a substitute for the more expensive bases DBU and DBN.[4] Since it is highly water-soluble, it is easily removed from mixtures in organic solvents. It is also used as a base-catalyst in the production of polyurethane.[5]
References
- ↑ "1,1,3,3-tetramethylguanidine - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 10 April 2012.
- ↑ Kaupmees, K., Trummal, A., Leito, I. (2014). "Basicities of Strong Bases in Water: A Computational Study". Croat. Chem. Acta 87: 385–395. doi:10.5562/cca2472.
- ↑ Rodima, Toomas; Leito, I. (2002). "Acid-Base Equilibria in Nonpolar Media. 2. Self-Consistent Basicity Scale in THF Solution Ranging from 2-Methoxypyridine to EtP1(pyrr) Phosphazene". J. Org. Chem. 67 (6): 1873–1881. doi:10.1021/jo016185p.
- 1 2 Ishikawa, T.; Kumamoto, T. (2006). "Guanidines in Organic Synthesis". Synthesis 2006 (5): 737–752. doi:10.1055/s-2006-926325.
- ↑ Geoghegan, J. T.; Roth, R. W. (2003). "Catalytic effects of 1,1,3,3-tetramethylguanidine for isocyanate reactions". J. Appl. Polymer Sci. 9 (3): 1089–1093. doi:10.1002/app.1965.070090325.
This article is issued from Wikipedia - version of the Tuesday, July 21, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.