Carboxyglutamic acid
 | |
| Names | |
|---|---|
| IUPAC name
3-Aminopropane-1,1,3-tricarboxylic acid | |
| Other names
γ-Carboxyglutamate | |
| Identifiers | |
| 53445-96-8 | |
| ChemSpider | 37241 |
| Jmol interactive 3D | Image |
| PubChem | 40772 |
| |
| |
| Properties | |
| C6H9NO6 | |
| Molar mass | 191.14 g/mol |
| Density | 1.649 g/mL |
| Boiling point | 418 °C (784 °F; 691 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Carboxyglutamic acid (or the conjugate base, carboxyglutamate), is an uncommon amino acid introduced into proteins by a post-translational carboxylation of glutamic acid residues. This modification is found, for example, in clotting factors and other proteins of the coagulation cascade. This modification introduces an affinity for calcium ions. In the blood coagulation cascade, Vitamin K is required to introduce gamma-carboxylation of clotting factors II, VII, IX, X and protein Z.[1]
Synthesis
In the biosynthesis of 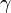 -carboxyglutamic acid, the
-carboxyglutamic acid, the 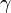 -proton on glutamic acid is abstracted, and CO2 is subsequently added. The reaction intermediate is a
-proton on glutamic acid is abstracted, and CO2 is subsequently added. The reaction intermediate is a 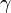 -glutamyl carbanion.
-glutamyl carbanion.
This reaction is catalyzed by a carboxylase that requires Vitamin K as its cofactor. It is not exactly known how Vitamin K participates, but it is hypothesized that a free cysteine residue in the carboxylase converts vitamin K into an active strong base that in turn abstracts a hydrogen from glutamic acid's 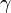 -carbon. Then CO2 is added to the
-carbon. Then CO2 is added to the 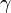 -carbon to form
-carbon to form 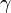 -carboxyglutamic acid. [2]
-carboxyglutamic acid. [2]
Gamma-carboxyglutamic acid-rich (GLA) domain
A number of gamma-carboxyglutamate (Gla) residues are present in the Gamma-carboxyglutamic acid-rich (GLA) domain. This GLA domain is known to be found in several proteins, including coagulation factors X, VII, IX, and XIV, vitamin K-dependent protein S and Z, prothrombin, transthyretin, osteocalcin, matrix Gla protein (MGP), inter-alpha trypsin inhibitor heavy chain H2, and growth arrest-specific protein 6 (Gas6). The Gla domain is responsible for the high-affinity binding of calcium ions.[3]
Role in Coagulation
Gamma-carboxyglutamic acid residues play an important role in coagulation. The high-affinity calcium binding sites in the GLA domain of factor IX, which is a serine protease of the coagulation system, were found to partially mediate the binding of factor IXa to platelets and in factor-X activation.[4] In addition, upon mechanical injury to the blood vessel wall, a cell-associated tissue factor becomes exposed and initiates a series of enzymatic reactions localized on a membrane surface generally provided by cells and accumulating platelets. Gla residues partly govern the activation and binding of circulating blood-clotting enzymes and zymogens to this exposed cell membrane surface. Specifically, gla residues are needed in calcium binding and in exposing hydrophobic membrane binding regions to the cell bilayer. Lack of these gla residues results in impaired coagulation or even anticoagulation, which may lead to bleeding diathesis or thrombosis.[5]
References
- ↑ J Stenflo, and J W Suttie. "Vitamin K-Dependent Formation of γ-Carboxyglutamic Acid". Annual Review of Biochemistry 46: 157–172. doi:10.1146/annurev.bi.46.070177.001105.
- ↑ Furie, Bruce; Bouchard, Beth A.; Furie, Barbara C. (1999-03-15). "Vitamin K-Dependent Biosynthesis of γ-Carboxyglutamic Acid". Blood 93 (6): 1798–1808. ISSN 0006-4971. PMID 10068650.
- ↑ "Gamma-carboxyglutamic acid-rich (GLA) domain (IPR000294) < InterPro < EMBL-EBI". www.ebi.ac.uk. Retrieved 2015-12-22.
- ↑ Rawala-Sheikh, R.; Ahmad, S. S.; Monroe, D. M.; Roberts, H. R.; Walsh, P. N. (1992-01-15). "Role of gamma-carboxyglutamic acid residues in the binding of factor IXa to platelets and in factor-X activation". Blood 79 (2): 398–405. ISSN 0006-4971. PMID 1730085.
- ↑ Kalafatis, M.; Egan, J. O.; van't Veer, C.; Mann, K. G. (1996-01-01). "Regulation and regulatory role of gamma-carboxyglutamic acid containing clotting factors". Critical Reviews in Eukaryotic Gene Expression 6 (1): 87–101. ISSN 1045-4403. PMID 8882309.