beta-Hydroxybutyric acid
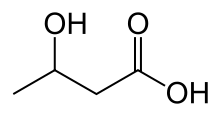 | |
| Names | |
|---|---|
| IUPAC name
3-Hydroxybutanoic acid | |
| Identifiers | |
| 300-85-6 | |
| ChEBI | CHEBI:20067 |
| ChEMBL | ChEMBL1162496 |
| ChemSpider | 428 |
| 1593 | |
| Jmol interactive 3D | Image Image |
| MeSH | beta-Hydroxybutyrate |
| PubChem | 441 |
| |
| |
| Properties | |
| C4H8O3 | |
| Molar mass | 104.11 g·mol−1 |
| Appearance | white solid |
| Melting point | 44-46 |
| Related compounds | |
| Other anions |
hydroxybutyrate |
| Related carboxylic acids |
propionic acid lactic acid 3-hydroxypropanoic acid malonic acid butyric acid hydroxypentanoic acid |
| Related compounds |
erythrose threose 1,2-butanediol 1,3-butanediol 2,3-butanediol 1,4-butanediol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
beta-Hydroxybutyric acid (also known as beta-hydroxybutyrate, 3-hydroxybutyric acid or 3-hydroxybutyrate) is an organic compound with the formula CH3CH(OH)CH2CO2H. It is a beta hydroxy acid. It is a chiral compound having two enantiomers, D-3-hydroxybutyric acid and L-3-hydroxybutyric acid. Its oxidized and polymeric derivatives occur widely in nature.
Biosynthesis
In humans, beta-hydroxybutyrate is synthesized in the liver from acetoacetate, the first ketone produced in the fasting state. The biosynthesis is catalyzed by the enzyme beta-hydroxybutyrate dehydrogenase.
Although not a ketone itself, the concentration of beta-hydroxybutyrate, like that of other ketone bodies, is raised in ketosis. This elevated beta-hydroxybutyrate level seen in ketosis is naturally expected due to the fact that, as mentioned above, it is formed from acetoacetate. The compound can be used as an energy source by the brain when blood glucose is low.[1] Diabetic patients can have their ketone levels tested via urine or blood to indicate diabetic ketoacidosis. In alcoholic ketoacidosis, this ketone body is produced in greatest concentration. Both types of ketoacidosis result in an increase beta-hydroxybutyrate to oxaloacetate ratio, resulting in TCA cycle stalling and shifting of glucose towards ketone body production.
Laboratory and industrial chemistry
beta-Hydroxybutyric acid is the precursor to polyesters, which are biodegradable plastics. Known as poly(3-hydroxybutyrate), this polymer is also produced biologically by the bacteria Alcaligenes eutrophus.[2]
beta-Hydroxybutyrate can be extracted from poly(3-hydroxybutyrate) by acid hydrolysis.[3]
See also
References
- ↑ O. E. Owen; et al. (1967). "Brain Metabolism during Fasting". The Journal of Clinical Investigation 46 (10): 1589–1595. doi:10.1172/JCI105650. PMC 292907. PMID 6061736.
- ↑ Yoshiharu Doi, Masao Kunioka, Yoshiyuki Nakamura, Kazuo Soga (1988). "Nuclear magnetic resonance studies on unusual bacterial copolyesters of 3-hydroxybutyrate and 4-hydroxybutyrate". Macromolecules 21 (9): 2722–2727. doi:10.1021/ma00187a012.
- ↑ Dieter Seebach, Albert K. Beck, Richard Breitschuh, and Kurt Job "Direct Degradation of the Biopolymer Poly[(R)-3-Hydroxybutrric Acid to (R)-3-Hydroxybutanoic Acid and Its Methyl Ester" Org. Synth. 1993, 71, 39. doi:10.15227/orgsyn.071.0039
| ||||||||||||||||||||||||||||||||||||||||||||||||||||