Zosuquidar
 | |
| Systematic (IUPAC) name | |
|---|---|
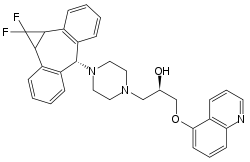
| (2R)-1-{4-[(1aR,10bS)-1,1-difluoro-1,1a,6,10b-tetrahydrodibenzo[a,e]cyclopropa[c][7]annulen-6-yl}-3-(quinolin-5-yloxy)propan-2-ol | |
| Clinical data | |
| Identifiers | |
|
167354-41-8 | |
| None | |
| PubChem | CID 153997 |
| ChemSpider |
24599682 |
| UNII |
AB5K82X98Y |
| KEGG |
D06387 |
| ChEMBL |
CHEMBL444172 |
| Chemical data | |
| Formula | C32H31F2N3O2 |
| 527.61 g/mol | |
|
SMILES
| |
| |
| | |
Zosuquidar is an experimental antineoplastic drug currently under development. It has completed Phase 3 clinical trials in the United States. It inhibits P-glycoproteins. Other drugs with this mechanism include tariquidar and laniquidar. P-glycoproteins are trans-membrane proteins that pump foreign substances out of cells in an ATP dependent fashion. Cancers overexpressing P-glycoproteins are able to pump out therapeutic molecules before they are able to reach their target, effectively making the cancer multi-drug resistant. Zosuquidar inhibits P-glycoproteins, inhibiting the efflux pump and restoring sensitivity to chemotherapeutic agents.