Zinc sulfate
 | |
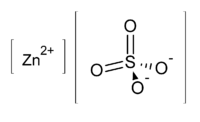 | |
| Names | |
|---|---|
| IUPAC name
Zinc sulfate | |
| Other names
White vitriol Goslarite | |
| Identifiers | |
| ATC code | A12 |
| 7733-02-0 7446-19-7 (monohydrate) 13986-24-8 (hexahydrate) 7446-20-0 (heptahydrate) | |
| ChEBI | CHEBI:35176 |
| ChEMBL | ChEMBL1200929 |
| ChemSpider | 22833 |
| EC number | 231-793-3 |
| |
| Jmol-3D images | Image |
| PubChem | 24424 |
| RTECS number | ZH5260000 |
| |
| UNII | 0J6Z13X3WO |
| UN number | 3077 |
| Properties | |
| ZnSO4 | |
| Molar mass | 161.47 g/mol (anhydrous) 179.47 g/mol (monohydrate) 287.53 g/mol (heptahydrate) |
| Appearance | white powder |
| Odor | odorless |
| Density | 3.54 g/cm3 (anhydrous) 2.072 g/cm3 (hexahydrate) |
| Melting point | 680 °C (1,256 °F; 953 K) decomposes (anhydrous) 100 °C (heptahydrate) 70 °C, decomposes (hexahydrate) |
| Boiling point | 740 °C (1,360 °F; 1,010 K) (anhydrous) 280 °C, decomposes (heptahydrate) |
| 57.7 g/100 mL, anhydrous (20 °C)[1] | |
| Solubility | alcohols |
| Refractive index (nD) |
1.658 (anhydrous), 1.4357 (heptahydrate) |
| Thermochemistry | |
| Std molar entropy (S |
120 J·mol−1·K−1[2] |
| Std enthalpy of formation (ΔfH |
−983 kJ·mol−1[2] |
| Hazards | |
| MSDS | ICSC 1698 |
| EU Index | 030-006-00-9 |
| EU classification | Harmful (Xn) Dangerous for the environment (N) |
| R-phrases | R22, R41, R50/53 |
| S-phrases | (S2), S22, S26, S39, S46, S60, S61 |
| Flash point | Non-flammable |
| Related compounds | |
| Other cations |
Cadmium sulfateManganese sulfate |
| Related compounds |
Copper(II) sulfate |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Zinc sulfate is the inorganic compound with the formula ZnSO4 as well as any of three hydrates. It was historically known as "white vitriol". It is a colorless solid that is a common source of soluble zinc ions.[3]
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[4]
Production and reactivity
Zinc sulfate is produced by treating zinc with aqueous sulfuric acid:
- Zn + H2SO4 + 7 H2O → ZnSO4(H2O)7 + H2
Pharmaceutical grade zinc sulfate is produced from high purity zinc oxide:
- ZnO + H2SO4 + 6 H2O → ZnSO4(H2O)7
In the laboratory, it can also be prepared by adding solid zinc to a copper(II) sulfate solution:
- Zn + CuSO4 → ZnSO4 + Cu
In aqueous solution, all forms of zinc sulfate behave identically. These aqueous solutions consist of the metal aquo complex [Zn(H2O)6]2+ and SO42− ions. Barium sulfate forms when these solutions are treated with solutions of barium ions:
- ZnSO4 + BaCl2 → BaSO4 + ZnCl2
With a reduction potential of -0.76, zinc(II) reduces only with difficulty.
When heated over 680 C, zinc sulfate decomposes into sulfur dioxide gas and zinc oxide fume, both of which are hazardous.[5]
Solubility only applies in acidic solutions.
Applications
The hydrates, especially the heptahydrate, are the primary forms used commercially. The main application is as a coagulant in the production of rayon. It is also a precursor to the pigment lithopone. Zinc sulfate is used to supply zinc in animal feeds, fertilizers, and agricultural sprays. Zinc sulfate, like many zinc compounds, can be used to control moss growth on roofs.[6] It is used as in electrolytes for zinc plating, as a mordant in dyeing, as a preservative for skins and leather and in medicine as an astringent and emetic.[3]
Minerals
As a mineral ZnSO4·7H2O is known as goslarite. Zinc sulfate occurs as several other minor minerals Zinc-melanterite (Zn,Cu,Fe)SO4·7H2O (structurally different from goslarite). Lower hydrates of zinc sulfate are rarely found in nature: (Zn,Fe)SO4·6H2O (bianchite ), (Zn,Mg)SO4·4H2O (boyleite), and (Zn,Mn)SO4·H2O (gunningite).
Safety
Zinc sulfate powder is an eye irritant. Ingestion of trace amounts is considered safe, and zinc sulfate is added to animal feed as a source of essential zinc, at rates of up to several hundred milligrams per kilogram of feed. Excess ingestion results in acute stomach distress, with nausea and vomiting appearing at 2-8 mg/Kg of body weight. [7]
References
- ↑ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ↑ 2.0 2.1 Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 0-618-94690-X.
- ↑ 3.0 3.1 Dieter M. M. Rohe, Hans Uwe Wolf "Zinc Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a28 537
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ "Zinc Sulphate Zinc Sulfate MSDS Sheet of Manufacturers". Mubychem.com. 2013-05-05. Retrieved 2013-06-08.
- ↑ "Moss on Roofs," Community Horticultural Fact Sheet #97, Washington State University King County Extension,
- ↑ European Food Safety Authority (EFSA), "Scientific Opinion on safety and efficacy of zinc compounds (E6) as feed additives for all animal species: Zinc sulphate monohydrate", Feb 2012
| ||||||||||||||
| Salts and the ester of the Sulfate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2SO4 | He | ||||||||||||||||||
| Li2SO4 | BeSO4 | B | (RO)2SO3 | (NH4)2SO4 N2H6SO4 (NH3OH)2SO4 |
O | F | Ne | ||||||||||||
| Na2SO4 NaHSO4 |
MgSO4 | Al2(SO4)3 | Si | P | SO42− | Cl | Ar | ||||||||||||
| K2SO4 KHSO4 |
CaSO4 | Sc2(SO4)3 | Ti(SO4)2 | V2(SO4)3 VOSO4 |
CrSO4 Cr2(SO4)3 |
MnSO4 | FeSO4 Fe2(SO4)3 |
CoSO4, Co2(SO4)3 |
NiSO4 | CuSO4 | ZnSO4 | Ga2(SO4)3 | Ge | As | Se | Br | Kr | ||
| Rb2SO4 | SrSO4 | Y | Zr(SO4)2 | Nb | Mo | Tc | Ru | Rh | PdSO4 | Ag2SO4 | CdSO4 | In2(SO4)3 | SnSO4 | Sb2(SO4)3 | Te | I | Xe | ||
| Cs2SO4 | BaSO4 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2SO4, HgSO4 |
Tl2SO4 | PbSO4 | Bi2(SO4)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | |||
| ↓ | |||||||||||||||||||
| La | Ce2(SO4)3 Ce(SO4)2 |
Pr2(SO4)3 | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb2(SO4)3 | Lu | |||||
| Ac | Th | Pa | U(SO4)2 UO2SO4 |
Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||