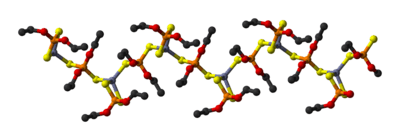Zinc dithiophosphate
2.png)
Zinc dialkyldithiophosphates (often referred to as ZDDP) are a family of coordination compounds developed in the 1940s[1] that feature zinc bound to the anion of dithiophosphoric acid. These uncharged compounds are not salts. They are soluble in nonpolar solvents, and the longer chain derivatives easily dissolve in mineral and synthetic oils used as lubricants. They come under CAS number
The alkyl groups can be branched and linear alkanes between 1-14 carbons length, 2-butyl, pentyl, hexyl, 1,3-dimethylbutyl, heptyl, octyl, isooctyl (2-ethylhexyl), 6-methylheptyl, 1-methylpropyl, dodecylphenyl, and others. A mix of zinc dialkyl(C3-C6)dithiophosphates come under CAS number
Coordination chemistry
These species are produced in two steps. First phosphorus pentasulfide is treated with suitable alcohols to give the dithiophosphoric acid. A wide variety of alcohols can be employed, which allows the lipophilicity of the final zinc product to be fine-tuned. The resulting dithiophosphate is then neutralized by adding zinc oxide:
- 2 (RO)2PS2H + ZnO → Zn[(S2P(OR)2]2 + H2O
In Zn[(S2P(OR)2]2, the zinc is tetrahedral. This monomeric compound also exists in equilibrium with dimers and oligomers caused by opening of the four-membered ZnS2P ring. Partial hydrolysis gives the cluster Zn4O[(S2P(OR)2]6, which adopts the structure seen for basic zinc acetate.
Zinc diethyldithiophosphate, Zn[(S2P(OEt)2]2, is a polymeric solid consisting of linear chains.[3]
Applications
The main use of ZDDP is in anti-wear additives to lubricants such as greases, gear oils, and motor oils, which often contain less than 1% of this additive. It has been reported that zinc and phosphorus emissions may damage catalytic converters and standard formulations of lubricating oils for gasoline engines now have reduced amounts of the additive, though diesel engine oils remain at higher levels.[4] Crankcase oils with reduced ZDDP have been cited as causing damage to, or failure of, classic/collector car flat tappet camshafts and lifters which undergo very high boundary layer pressures and/or shear forces at their contact faces, and in other regions such as big-end/main bearings, and piston rings and pins. Roller camshafts are more commonly used to reduce camshaft lobe friction in modern engines. There are additives, such as STP(R) Oil Treatment, and some racing oils such as PurOl, Brad Penn and Valvoline VR-1, which are available in the retail market with the necessary amount of ZDDP for engines using increased valve spring pressures. The same ZDDP compounds serve also as corrosion inhibitors and antioxidants.
Naming
These compounds are widely used and correspondingly have numerous names, including ZDDP, ZnDTP, and ZDP.
References
- ↑ H. Spikes "The history and mechanisms of ZDDP" Tribology Letters, Vol. 17, No. 3, October 2004. doi:10.1016/S0301-679X(01)00028-7.
- ↑ Allyson M. Barnes, Keith D. Bartle and Vincent R. A. Thibo "A review of zinc dialkyldithiophosphates (ZDDPS): characterisation and role in the lubricating oil" Tribology International 2001, Pages 389-395. doi:10.1023/B:TRIL.0000044495.26882.b5.
- ↑ T. Ito, T. Igarashi, H. Hagihara (1969). "The crystal structure of metal diethyldithiophosphates. I. Zinc diethyldithiophosphate". Acta Cryst. B25 (11): 2303–2309. doi:10.1107/S0567740869005619.
- ↑ "ZDDP Engine Oil - The Zinc Factor". Mustang Monthly. Retrieved 2009-09-19.