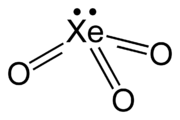
Xenon trioxide
 | |
 | |
| Names | |
|---|---|
| IUPAC names
Xenon trioxide Xenon(VI) oxide | |
| Other names
Xenic anhydride | |
| Identifiers | |
| 13776-58-4 | |
| ChemSpider | 21106493 |
| |
| Jmol-3D images | Image |
| |
| Properties | |
| XeO3 | |
| Molar mass | 179.288 g/mol |
| Appearance | colourless crystalline solid |
| Density | 4.55 g/cm3, solid |
| Melting point | 25 °C (77 °F; 298 K) Violent decomposition |
| Soluble (with reaction) | |
| Structure | |
| Molecular shape | trigonal pyramidal (C3v) |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH |
402 kJ·mol−1[1] |
| Hazards | |
| EU classification | not listed |
| NFPA 704 | |
| Related compounds | |
| Related compounds |
Xenon tetroxide Xenic acid |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly (and xenon), accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials. When it detonates, it releases xenon and oxygen gas.
Chemistry
Xenon trioxide is a strong oxidising agent and can oxidise. most substances that are at all oxidisable. However, it is slow-acting and this reduces its usefulness.[2]
Above 25 °C, xenon trioxide is very prone to violent explosion:
- 2 XeO3 → 2 Xe + 3 O2
When it dissolves in water, an acidic solution of xenic acid is formed:
- XeO3 (aq) + H2O → H2XeO4
 H+ + HXeO4−
H+ + HXeO4−
This solution is stable at room temperature and lacks the explosive properties of xenon trioxide. It oxidises carboxylic acids quantitatively to carbon dioxide and water.[3]
Alternatively, it dissolves in alkaline solutions to form xenates. The HXeO−
4 anion is the predominant species in xenate solutions.[4] These are not stable and begin to disproportionate into perxenates (+8 oxidation state) and xenon and oxygen gas.[5] Solid perxenates containing XeO4−
6 have been isolated by reacting XeO
3 with an aqueous solution of hydroxides. Xenon trioxide reacts with inorganic fluorides such as KF, RbF, or CsF to form stable solids of the form MXeO
3F.[6]
Physical properties
Hydrolysis of xenon hexafluoride or xenon tetrafluoride yields a solution from which colorless XeO3 crystals can be obtained by evaporation.[7] The crystals are stable for days in dry air, but readily absorb water from humid air to form a concentrated solution. The crystal structure is orthorhombic with a = 6.163, b = 8.115, c = 5.234 Å and 4 molecules per unit cell. The density is 4.55 g/cm3.[8]
 |  | 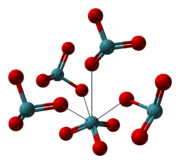 |
| the crystal structure of XeO3 |
Safety
XeO3 should be handled with great caution. Samples have detonated when undisturbed at room temperature. Dry crystals react explosively with cellulose.[8][9]
References
- ↑ Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 0-618-94690-X.
- ↑ Greenwood, N.; Earnshaw, A. (1997). Chemistry of the Elements. Oxford: Butterworth-Heinemann.
- ↑ Jaselskis B.; Krueger R. H. (July 1966). "Titrimetric determination of some organic acids by xenon trioxide oxidation". Talanta 13 (7): 945–949. doi:10.1016/0039-9140(66)80192-3. PMID 18959958.
- ↑ Peterson, J. L.; Claassen, H. H.; Appelman, E. H. (March 1970). "Vibrational spectra and structures of xenate(VI) and perxenate(VIII) ions in aqueous solution". Inorganic Chemistry 9 (3): 619–621. doi:10.1021/ic50085a037.
- ↑ W. Henderson (2000). Main group chemistry. Great Britain: Royal Society of Chemistry. pp. 152–153. ISBN 0-85404-617-8.
- ↑ Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. p. 399. ISBN 0-12-352651-5.
- ↑ John H. Holloway; Eric G. Hope (1998). A. G. Sykes, ed. Recent Advances in Noble-gas Chemistry. Advances in Inorganic Chemistry, Volume 46. Academic Press. p. 65. ISBN 0-12-023646-X.
- ↑ 8.0 8.1 Templeton, D. H.; Zalkin, A.; Forrester, J. D.; Williamson, S. M. (1963). "Crystal and Molecular Structure of Xenon Trioxide". Journal of the American Chemical Society 85 (6): 817. doi:10.1021/ja00889a037.
- ↑ Bartlett, N.; Rao, P. R. (1963). "Xenon Hydroxide: an Experimental Hazard". Science 139 (3554): 506. Bibcode:1963Sci...139..506B. doi:10.1126/science.139.3554.506. PMID 17843880.
External links
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
