Wilkinson's catalyst
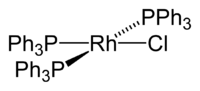 | |
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC names
(SP-4)chloridotris(triphenylphosphane) rhodium | |
| Other names
Rhodium(I) tris- (triphenylphosphine) chloride, Wilkinson's catalyst, Tris(triphenylphosphine)- rhodium chloride | |
| Identifiers | |
| 14694-95-2 | |
| EC number | 238-744-5 |
| Jmol-3D images | Image |
| PubChem | 84599 |
| RTECS number | none |
| |
| Properties | |
| C54H45ClP3Rh | |
| Molar mass | 925.22 g/mol |
| Appearance | red solid |
| Melting point | 245 to 250 °C (473 to 482 °F; 518 to 523 K) |
| insoluble in water | |
| Solubility in other solvents | benzene |
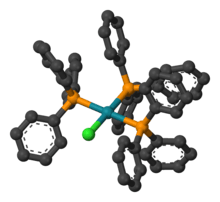
| Structure | |
| square planar | |
| Hazards | |
| Main hazards | none |
| R-phrases | none |
| S-phrases | S22 S24/25 |
| Related compounds | |
| Related compounds |
triphenylphosphine Pd(PPh3)4 IrCl(CO)[P(C6H5)3]2 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Wilkinson's catalyst is the common name for chlorotris(triphenylphosphine)rhodium(I), a coordination compound with the formula RhCl(PPh3)3 (Ph = phenyl). It is named after the chemist and Nobel Laureate, Sir Geoffrey Wilkinson who popularized its use.
Structure and basic properties
The compound is a square planar, 16-electron complex. It is usually obtained in the form of a red-violet crystalline solid from the reaction of rhodium(III) chloride with excess triphenylphosphine.[1] The synthesis is conducted in refluxing ethanol which helps with the reduction.[2][3] Triphenylphosphine serves as the reducing agent yielding triphenylphosphine oxide.
- RhCl3(H2O)3 + 4 PPh3 → RhCl(PPh3)3 + OPPh3 + 2 HCl + 2 H2O
Catalytic applications
Wilkinson's catalyst catalyzes the hydrogenation of alkenes.[4][5] The mechanism of this reaction involves the initial dissociation of one or two triphenylphosphine ligands to give 14- or 12-electron complexes, respectively, followed by oxidative addition of H2 to the metal. Subsequent π-complexation of alkene, intramolecular hydride transfer (olefin insertion), and reductive elimination results in extrusion of the alkane product, e.g.:
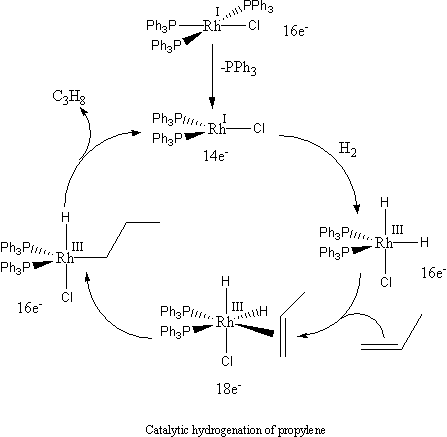
Other applications of Wilkinson's catalyst includes the catalytic hydroboration of alkenes with catecholborane and pinacolborane,[6] and the selective 1,4-reduction of α, β-unsaturated carbonyl compounds in concert with triethylsilane.[7] When the triphenylphosphine ligands are replaced by chiral phosphines (e.g., chiraphos, DIPAMP, DIOP), the catalyst becomes chiral and converts prochiral alkenes into enantiomerically enriched alkanes via the process called asymmetric hydrogenation.[8]
Other reactions of RhCl(PPh3)3
RhCl(PPh3)3 reacts with CO to give trans-RhCl(CO)(PPh3)2, which is structurally analogous to Vaska's complex (but much less reactive). The same complex arises from the decarbonylation of aldehydes:
- RhCl(PPh3)3 + RCHO → RhCl(CO)(PPh3)2 + RH + PPh3
Upon stirring in benzene solution, RhCl(PPh3)3 converts to the poorly soluble red-colored species Rh2Cl2(PPh3)4. This conversion further demonstrates the lability of the triphenylphosphine ligands.
See also
References
- ↑ Bennett, M. A.; Longstaff, P. A. Complexes of Rhodium(I) with Triphenylphosphine. Chem. Ind. (London) 1965, p. 846.
- ↑ Osborn, J. A.; Jardine, F. H.; Young, J. F.; Wilkinson, G. (1966). "The Preparation and Properties of Tris(triphenylphosphine)halogenorhodium(I) and Some Reactions Thereof Including Catalytic Homogeneous Hydrogenation of Olefins and Acetylenes and Their Derivatives". Journal of the Chemical Society A: 1711–1732. doi:10.1039/J19660001711.
- ↑ "Tris(triphenylphosphine)halorhodium(I)" J. A. Osborn, G. Wilkinson, Inorganic Syntheses, 1967, Volume 10, p. 67. doi:10.1002/9780470132418.ch12
- ↑ A. J. Birch, D. H. Williamson (1976). Organic Reactions 24: 1ff. Missing or empty
|title=(help) - ↑ B.R. James, Homogeneous Hydrogenation. John Wiley & Sons, New York, 1973.
- ↑ D. A. Evans, G. C. Fu and A. H. Hoveyda (1988). "Rhodium(I)-catalyzed hydroboration of olefins. The documentation of regio- and stereochemical control in cyclic and acyclic systems". J. Am. Chem. Soc. 110 (20): 6917–6918. doi:10.1021/ja00228a068.
- ↑ I. Ojima, T. Kogure (1972). "Selective reduction of α,β-unsaturated terpene carbonyl compounds using hydrosilane-rhodium(I) complex combinations". Tetrahedron Lett. 13 (49): 5035–5038. doi:10.1016/S0040-4039(01)85162-5.
- ↑ W. S. Knowles (2003). "Asymmetric Hydrogenations (Nobel Lecture 2001)". Advanced Synthesis and Catalysis 345 (12): 3–13. doi:10.1002/adsc.200390028.