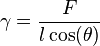Wilhelmy plate
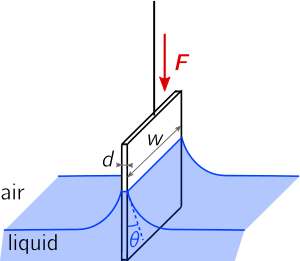
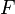 on the plate is proportionnal to the wetted perimeter,
on the plate is proportionnal to the wetted perimeter, 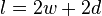 , and to the surface tension
, and to the surface tension 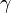 of the liquid-air interface.
of the liquid-air interface.A Wilhelmy plate is a thin plate that is used to measure equilibrium surface or interfacial tension at an air–liquid or liquid–liquid interface. In this method, the plate is oriented perpendicular to the interface, and the force exerted on it is measured. Based on the work of Ludwig Wilhelmy, this method finds wide use in the preparation and monitoring of Langmuir–Blodgett films.
Detailed description
The Wilhelmy plate consists of a thin plate usually on the order of a few square centimeters in area. The plate is often made from filter paper, glass or platinum which may be roughened to ensure complete wetting. In fact, the results of the experiment are irrelevant of the material used, as long as the material is wetted by the liquid.[1] The plate is cleaned thoroughly and attached to a scale or balance via a thin metal wire. The force on the plate due to wetting is measured via a tensiometer or microbalance and used to calculate the surface tension (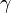 ) using the Wilhelmy equation:
) using the Wilhelmy equation:
where 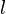 is the wetted perimeter (
is the wetted perimeter (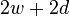 ;
; 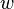 is the plate width and
is the plate width and 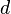 is the plate thickness) of the Wilhelmy plate and
is the plate thickness) of the Wilhelmy plate and 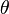 is the contact angle between the liquid phase and the plate. In practice the contact angle is rarely measured, instead either literature values are used, or complete wetting (
is the contact angle between the liquid phase and the plate. In practice the contact angle is rarely measured, instead either literature values are used, or complete wetting (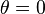 ) is assumed.
) is assumed.
Advantages and practice
Unlike a Du Noüy ring, no correction factors are required when calculating surface tensions when using the Wilhelmy plate, assuming a zero contact angle. In addition, because the plate is not moved during measurements, the Wilhelmy plate allows accurate determination of surface kinetics on a wide range of timescales and it displays low operator variance. In a typical plate experiment, the plate is lowered to the surface being analyzed until a meniscus is formed, and then raised so that the bottom edge of the plate lies on the plane of the undisturbed surface. If measuring a buried interface, the second (less dense) phase is then added on top of the undisturbed primary (denser) phase in such a way as to not disturb the meniscus. The force at equilibrium can then be used to determine the absolute surface or interfacial tension.
See also
Further reading
- Holmberg, K (ed.) Handbook of Applied Surface and Colloid Chemistry New York, Wiley and Sons: 2002. Vol. 2, p. 219