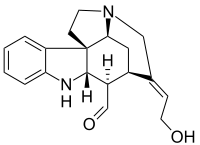
Wieland-Gumlich aldehyde
 | |
| Identifiers | |
|---|---|
| ChemSpider | 30668088 |
| |
| Jmol-3D images | Image |
| |
| Properties | |
| Molecular formula |
C19H22N2O2 |
| Molar mass | 310.39 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
The so-called Wieland-Gumlich aldehyde (6) is an indoline derived by chemical degradation from strychnine. This compound is of some commercial interest as a chemical intermediate. It was first synthesized in 4 steps from strychnine (1)[1][2][3] by Walter Gumlich and Koozoo Kaziro working in the laboratory of Heinrich Wieland. This degradation study was part of an attempt to elucidate the chemical structure of strychnine.
This degradation takes place through conversion of strychnine to the oxime 2 using amyl nitrite, Beckmann fragmentation of 2 to the carbamic acid 3 by use of thionyl chloride, decarboxylation of 3 to nitrile 4, and nucleophilic displacement of cyanide by barium hydroxide to give hemiacetal 5, which is in equilibrium with the Wieland-Gumlich aldehyde (6).
The Wieland-Gumlich aldehyde reverts to strychnine in a single reaction using malonic acid, acetic anhydride and sodium acetate in acetic acid.[4]
The Wieland-Gumlich aldehyde has been used in the industrial synthesis of alcuronium chloride (Alloferin) via dimerization.[5]
References
- ↑ Wieland, H.; Gumlich, W. (1932) Über einige neue Reaktionen der Strychnos - Alkaloide. XI Justus Liebigs Annalen der Chemie 494(1):191-200. (original report of fragmentation of the strychnine lactam ring)
- ↑ Wieland H.; Kaziro, K. (1933) Abbauversuche vom Isonitroso-strychnin aus. Über Strychnos-Alkaloide. XIII Justus Liebigs Annalen der Chemie 506(1):60–76. (definitive characterization of the Wieland-Gumlich aldehyde)
- ↑ Witkop B. (1992) Remembering Heinrich Wieland (1877-1957) Portrait of an Organic Chemist and Founder of Modern Biochemistry Med. Res. Rev. 12(3):195-274. (Witkop notes [p. 220] that "Wieland and Kaziro studied the Beckmann rearrangement of this oxime and the loss of hydrogen cyanide to yield an aldehyde, that should correctly be called Wieland-Kaziro aldehyde, but became known and accepted as Wieland-Gumlich aldehyde...")
- ↑ F. A. L. Anet, R. Robinson, Chem. Ind. (London) 1953, 245.
- ↑ Alkaloids: Nature's Curse or Blessing? Manfred Hesse. Wiley, 2002. See pp. 230-232. ISBN 978-3-90639-024-6