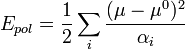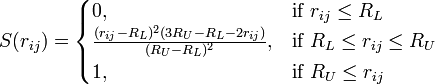Water model

In computational chemistry, classical water models are used for the simulation of water clusters, liquid water, and aqueous solutions with explicit solvent. The models are determined from quantum mechanics, molecular mechanics, experimental results, and these combinations. To imitate a specific nature of molecules, many types of model have been developed. In general, these can be classified by following three points; (i) the number of interaction points called site, (ii) whether the model is rigid or flexible, (iii) whether the model includes polarization effects.
An alternative to the explicit water models is to use an implicit solvation model, also known as a continuum model, an example of which would be the COSMO Solvation Model or the Polarizable continuum model (PCM) or a hybrid solvation model.[1]
Simple water models
The rigid models are known as the simplest water models which rely on non-bonded interactions. In these models, bonding interactions are implicitly treated by holonomic constraints. The electrostatic interaction is modeled using Coulomb's law and the dispersion and repulsion forces using the Lennard-Jones potential.[2][3] The potential for models such as TIP3P and TIP4P is represented by
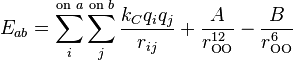
where kC, the electrostatic constant, has a value of 332.1 Å·kcal/mol in the units commonly used in molecular modeling; qi are the partial charges relative to the charge of the electron; rij is the distance between two atoms or charged sites; and A and B are the Lennard-Jones parameters. The charged sites may be on the atoms or on dummy sites (such as lone pairs). In most water models, the Lennard-Jones term applies only to the interaction between the oxygen atoms.
The figure below shows the general shape of the 3- to 6-site water models. The exact geometric parameters (the OH distance and the HOH angle) vary depending on the model.
2-site
A 2-site model of water based on the familiar three-site SPC model (see below) has been shown to predict the dielectric properties of water using site-renormalized molecular fluid theory.[4]
3-site
Three-site models have three interaction points corresponding to the three atoms of the water molecule. Each site has a point charge, and the site corresponding to the oxygen atom also has the Lennard-Jones parameters. Since 3-site models achieve a high computational efficiency, these are widely used for many applications of molecular dynamics simulations. Most of models use a rigid geometry matching that of actual water molecules. An exception is the SPC model, which assumes an ideal tetrahedral shape (HOH angle of 109.47°) instead of the observed angle of 104.5°.
The table below lists the parameters for some 3-site models.
| TIPS[5] | SPC[6] | TIP3P[7] | SPC/E[8] | |
|---|---|---|---|---|
| r(OH), Å | 0.9572 | 1.0 | 0.9572 | 1.0 |
| HOH, deg | 104.52 | 109.47 | 104.52 | 109.47 |
| A × 10−3, kcal Å12/mol | 580.0 | 629.4 | 582.0 | 629.4 |
| B, kcal Å6/mol | 525.0 | 625.5 | 595.0 | 625.5 |
| q(O) | −0.80 | −0.82 | −0.834 | −0.8476 |
| q(H) | +0.40 | +0.41 | +0.417 | +0.4238 |
The SPC/E model adds an average polarization correction to the potential energy function:
where μ is the dipole of the effectively polarized water molecule (2.35 D for the SPC/E model), μ0 is the dipole moment of an isolated water molecule (1.85 D from experiment), and αi is an isotropic polarizability constant, with a value of 1.608 × 10−40 F m2. Since the charges in the model are constant, this correction just results in adding 1.25 kcal/mol (5.22 kJ/mol) to the total energy. The SPC/E model results in a better density and diffusion constant than the SPC model.
The TIP3P model implemented in the CHARMM force field is a slightly modified version of the original. The difference lies in the Lennard-Jones parameters: unlike TIP3P, the CHARMM version of the model places Lennard-Jones parameters on the hydrogen atoms too, in addition to the one on oxygen. The charges are not modified.[9]
Flexible SPC water model

The flexible simple point charge water model (or Flexible SPC water model) is a re-parametrization of the three-site SPC water model.[10][11] The SPC model is rigid, whilst the flexible SPC model is flexible. In the model of Toukan and Rahman, the O-H stretching is made anharmonic and thus the dynamical behavior is well described. This is one of the most accurate three-center water models without taking into account the polarization. In molecular dynamics simulations it gives the correct density and dielectric permittivity of water.[12]
Flexible SPC is implemented in the MDynaMix and Abalone programs.
Other models
- Ferguson (flex. SPC)
- CVFF (flex.)
- MG (flexible and dissociative)MG model
4-site
The four-site models have four interaction points by adding one dummy atom near of the oxygen along the bisector of the HOH angle of the three-site models (labeled M in the figure). The dummy atom only has a negative charge. This model improves the electrostatic distribution around the water molecule. The first model to use this approach was the Bernal-Fowler model published in 1933, which may also be the earliest water model. However, the BF model doesn't reproduce well the bulk properties of water, such as density and heat of vaporization, and is therefore only of historical interest. This is a consequence of the parameterization method; newer models, developed after modern computers became available, were parameterized by running Metropolis Monte Carlo or molecular dynamics simulations and adjusting the parameters until the bulk properties are reproduced well enough.
The TIP4P model, first published in 1983, is widely implemented in computational chemistry software packages and often used for the simulation of biomolecular systems. There have been subsequent reparameterizations of the TIP4P model for specific uses: the TIP4P-Ew model, for use with Ewald summation methods; the TIP4P/Ice, for simulation of solid water ice; and TIP4P/2005, a general parameterization for simulating the entire phase diagram of condensed water.
Most of four-site water models use OH distance and HOH angle matching that of the water molecule in gas. An exception is OPC model on which no geometry constraints are imposed other than the fundamental C2v molecular symmetry of the water molecule. Instead, the point charges and their positions are optimized to best describe the electrostatics of the water molecule. OPC reproduces a comprehensive set of bulk properties more accurately than commonly used rigid n-site water models. OPC model is implemented in AMBER force field.
| BF[13] | TIPS2[14] | TIP4P[7] | TIP4P-Ew[15] | TIP4P/Ice[16] | TIP4P/2005[17] | OPC[18] | TIP4P-D[19] | |
|---|---|---|---|---|---|---|---|---|
| r(OH), Å | 0.96 | 0.9572 | 0.9572 | 0.9572 | 0.9572 | 0.9572 | 0.8724 | ? |
| HOH, deg | 105.7 | 104.52 | 104.52 | 104.52 | 104.52 | 104.52 | 103.6 | ? |
| r(OM), Å | 0.15 | 0.15 | 0.15 | 0.125 | 0.1577 | 0.1546 | 0.1594 | ? |
| A × 10−3, kcal Å12/mol | 560.4 | 695.0 | 600.0 | 656.1 | 857.9 | 731.3 | 865.1 | 904.7 |
| B, kcal Å6/mol | 837.0 | 600.0 | 610.0 | 653.5 | 850.5 | 736.0 | 858.1 | 900.0 |
| q(M) | −0.98 | −1.07 | −1.04 | −1.04844 | −1.1794 | −1.1128 | -1.3582 | ? |
| q(H) | +0.49 | +0.535 | +0.52 | +0.52422 | +0.5897 | +0.5564 | +0.6791 | +0.58 |
Note: The TIP4P-D manuscript is available ahead of publication, at which time these parameters should become known.
Others:
- TIP4PF (flexible)
5-site
The 5-site models place the negative charge on dummy atoms (labeled L) representing the lone pairs of the oxygen atom, with a tetrahedral-like geometry. An early model of these types was the BNS model of Ben-Naim and Stillinger, proposed in 1971, soon succeeded by the ST2 model of Stillinger and Rahman in 1974. Mainly due to their higher computational cost, five-site models were not developed much until 2000, when the TIP5P model of Mahoney and Jorgensen was published. When compared with earlier models, the TIP5P model results in improvements in the geometry for the water dimer, a more "tetrahedral" water structure that better reproduces the experimental radial distribution functions from neutron diffraction, and the temperature of maximum density of water. The TIP5P-E model is a reparameterization of TIP5P for use with Ewald sums.
| BNS[20] | ST2[20] | TIP5P[21] | TIP5P-E[22] | |
|---|---|---|---|---|
| r(OH), Å | 1.0 | 1.0 | 0.9572 | 0.9572 |
| HOH, deg | 109.47 | 109.47 | 104.52 | 104.52 |
| r(OL), Å | 1.0 | 0.8 | 0.70 | 0.70 |
| LOL, deg | 109.47 | 109.47 | 109.47 | 109.47 |
| A × 10−3, kcal Å12/mol | 77.4 | 238.7 | 544.5 | 554.3 |
| B, kcal Å6/mol | 153.8 | 268.9 | 590.3 | 628.2 |
| q(L) | −0.19562 | −0.2357 | −0.241 | −0.241 |
| q(H) | +0.19562 | +0.2357 | +0.241 | +0.241 |
| RL, Å | 2.0379 | 2.0160 | ||
| RU, Å | 3.1877 | 3.1287 |
Note, however, that the BNS and ST2 models do not use Coulomb's law directly for the electrostatic terms, but a modified version that is scaled down at short distances by multiplying it by the switching function S(r):
Therefore the RL and RU parameters only apply to BNS and ST2.
6-site
A 6-site model that combines all the sites of the 4- and 5-site models was developed by Nada and van der Eerden.[23] Originally designed to study water/ice systems, however has a very high melting temperature[24]
Other
- The effect of explicit solute model on solute behavior in bimolecular simulations has been also extensively studied. It was shown that explicit water models affected the specific solvation and dynamics of unfolded peptides while the conformational behavior and flexibility of folded peptides remained intact.[25]
- MB model. A more abstract model resembling the Mercedes-Benz logo that reproduces some features of water in two-dimensional systems. It is not used as such for simulations of "real" (i.e., three-dimensional) systems, but it is useful for qualitative studies and for educational purposes.[26]
- Coarse-grained models. One- and two-site models of water have also been developed.[27] In coarse grain models, each site can represent several water molecules.
Computational cost
The computational cost of a water simulation increases with the number of interaction sites in the water model. The CPU time is approximately proportional to the number of interatomic distances that need to be computed. For the 3-site model, 9 distances are required for each pair of water molecules (every atom of one molecule against every atom of the other molecule, or 3 × 3). For the 4-site model, 10 distances are required (every charged site with every charged site, plus the O-O interaction, or 3 × 3 + 1). For the 5-site model, 17 distances are required (4 × 4 + 1). Finally, for the 6-site model, 26 distances are required (5 × 5 + 1).
When using rigid water models in molecular dynamics, there is an additional cost associated with keeping the structure constrained, using constraint algorithms (although with bond lengths constrained it is often possible to increase the time step).
See also
- Water (properties)
- Water (data page)
- Water dimer
- Force field
- Force field implementation
- Molecular mechanics
- Molecular modelling
- Software for molecular mechanics modeling
References
- ↑ Skyner, R.; McDonagh, J. L., Groom, C. R., van Mourik, T., Mitchell, J. B. O. (2015). "A Review of Methods for the Calculation of Solution Free Energies and the Modelling of Systems in Solution". RSC, PCCP. doi:10.1039/C5CP00288E.
- ↑ Allen and Tildesley. (1989). Computer Simulation of Liquids.
- ↑ Kirby BJ. Micro- and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices.
- ↑ Dyer KM; Perkyns JS; Stell G; Pettitt BM. Site-Renormalized molecular fluid theory: on the utility of a two-site model of water. Mol. Phys. 2009, 107, 423-431.
- ↑ Jorgensen, W. L. Quantum and statistical mechanical studies of liquids. 10. Transferable intermolecular potential functions for water, alcohols, and ethers. Application to liquid water. J. Am. Chem. Soc. 1981, 103, 335-340.
- ↑ H.J.C. Berendsen, J.P.M. Postma, W.F. van Gunsteren, and J. Hermans, In Intermolecular Forces, edited by B. Pullman (Reidel, Dordrecht, 1981), p. 331.
- ↑ 7.0 7.1 Jorgensen, W. L.; Chandrasekhar, J.; Madura, J. D.; Impey, R. W.; Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys 1983, 79, 926-935. doi:10.1063/1.445869
- ↑ H. J. C. Berendsen, J. R. Grigera, and T. P. Straatsma. The Missing Term in Effective Pair Potentials. J. Phys. Chem 1987, 91, 6269-6271. doi:10.1021/j100308a038
- ↑ MacKerell, A. D., Jr.; Bashford, D.; Bellott, R. L.; Dunbrack, R. L., Jr.; Evanseck, J. D.; Field, M. J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; Joseph-McCarthy, D.; Kuchnir, L.; Kuczera, K.; Lau, F. T. K.; Mattos, C.; Michnick, S.; Ngo, T.; Nguyen, D. T.; Prodhom, B.; Reiher, W. E., III; Roux, B.; Schlenkrich, M.; Smith, J. C.; Stote, R.; Straub, J.; Watanabe, M.; Wiorkiewicz-Kuczera, J.; Yin, D.; Karplus, M. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. 1998, 102, 3586-3616. doi:10.1021/jp973084f
- ↑ K. Toukan and A. Rahman (1985). "Molecular-dynamics study of atomic motions in water". Physical Review B 31: 2643–2648. Bibcode:1985PhRvB..31.2643T. doi:10.1103/PhysRevB.31.2643.
- ↑ H. J. C. Berendsen, J. R. Grigera, and T. P. Straatsma (1987). "The missing term in effective pair potentials". Journal of Physical Chemistry 91: 6269–6271. doi:10.1021/j100308a038.
- ↑ M. Praprotnik, D. Janezic and J. Mavri (2004). "Temperature Dependence of Water Vibrational Spectrum: A Molecular Dynamics Simulation Study". Journal of Physical Chemistry A 108: 11056–11062. doi:10.1021/jp046158d.
- ↑ Bernal, J. D.; Fowler, R.H. J. Chem. Phys. 1933, 1, 515. doi:10.1063/1.1749327
- ↑ Jorgensen, W. L. Revised TIPS for simulations of liquid water and aqueous solutions. J. Chem. Phys 1982, 77, 4156-4163. doi:10.1063/1.444325
- ↑ H. W. Horn, W. C. Swope, J. W. Pitera, J. D. Madura, T. J. Dick, G. L. Hura, and T. Head-Gordon. Development of an improved four-site water model for biomolecular simulations: TIP4P-Ew. J. Chem. Phys. 2004, 120, 9665-9678. doi:10.1063/1.1683075
- ↑ J. L. F. Abascal, E. Sanz, R. García Fernández, and C. Vega. A potential model for the study of ices and amorphous water: TIP4P/Ice. J. Chem. Phys. 2005, 122, 234511. doi:10.1063/1.1931662
- ↑ J. L. F. Abascal and C. Vega. A general purpose model for the condensed phases of water: TIP4P/2005. J. Chem. Phys. 2005, 123, 234505. doi:10.1063/1.2121687
- ↑ S. Izadi, R. Anandakrishnan, and A. V. Onufriev. Building Water Models: A Different Approach. The Journal of Physical Chemistry Letters 2014, 5 (21), 3863–3871. doi:10.1021/jz501780a
- ↑ Piana, Stefano; Donchev, Alexander G.; Robustelli, Paul; Shaw, David E. (2015). "Water Dispersion Interactions Strongly Influence Simulated Structural Properties of Disordered Protein States". The Journal of Physical Chemistry B: 150312133835006. doi:10.1021/jp508971m. ISSN 1520-6106.
- ↑ 20.0 20.1 F.H. Stillinger, A. Rahman, Improved simulation of liquid water by molecular dynamics. J. Chem. Phys. 1974, 60, 1545-1557. doi:10.1063/1.1681229
- ↑ Mahoney, M. W.; Jorgensen, W. L. A five-site model liquid water and the reproduction of the density anomaly by rigid, non-polarizable models. J. Chem. Phys. 2000, 112, 8910-8922. doi:10.1063/1.481505
- ↑ Rick, S. W. A reoptimization of the five-site water potential (TIP5P) for use with Ewald sums. J. Chem. Phys. 2004, 120, 6085-6093. doi:10.1063/1.1652434
- ↑ H. Nada, J.P.J.M. van der Eerden, J. Chem. Phys. 2003, 118, 7401. doi:10.1063/1.1562610
- ↑ Abascal et al.doi:10.1063/1.2360276
- ↑ P. Florova, P. Sklenovsky, P. Banas, M. Otyepka. J. Chem. Theory Comput. 2010, 6, 3569–3579. doi:10.1021/ct1003687
- ↑ K. A. T. Silverstein, A. D. J. Haymet, and K. A. Dill. A Simple Model of Water and the Hydrophobic Effect. J. Am. Chem. Soc. 1998, 120, 3166-3175. doi:10.1021/ja973029k
- ↑ S. Izvekov, G. A. Voth. Multiscale coarse graining of liquid-state systems J. Chem. Phys. 2005, 123, 134105. doi:10.1063/1.2038787
| |||||||||||||||||||||||||