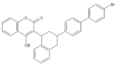
Vitamin K antagonist

Vitamin K antagonists (VKA) are a group of substances that reduce blood clotting by reducing the action of vitamin K. They are used as rodenticides and as anticoagulant medications in the prevention of thrombosis.
Mechanism of action
These drugs deplete the active form of the vitamin by inhibiting the enzyme vitamin K epoxide reductase and thus the recycling of the inactive vitamin K epoxide back to the active reduced form of vitamin K. The drugs are structurally similar to vitamin K and act as competitive inhibitors of the enzyme. The term "vitamin K antagonist" is a misnomer, as the drugs do not directly antagonise the action of vitamin K in the pharmacological sense, but rather the recycling of vitamin K.
Vitamin K is required for the proper production of certain proteins involved in the blood clotting process.
The action of this class of anticoagulants may be reversed by administering vitamin K for the duration of the anticoagulant's residence in the body, and the daily dose needed for reversal is the same for all drugs in the class. However, in the case of the second generation "super warfarins" intended to kill warfarin resistant rodents, the time of vitamin K administration may need to be prolonged to months, in order to combat the long residence time of the poison.[1]
The vitamin K antagonists can cause birth defects (teratogens).[2]
Coumadins (4-hydroxycoumarins)
Coumarins (more accurately 4-hydroxycoumarins) are the most commonly used VKAs.
In medicine, the most commonly used VKA is warfarin.[3] Warfarin was initially used as a rodenticide, but made the transition to pharmaceutical. Eventually some rodents developed resistance to it. The "second generation" VKAs for dedicated use as rodenticides are sometimes called "super warfarins." These VKAs are enhanced to kill warfarin-resistant rodents. The enhancement to the molecule takes the form of a larger lipophilic group to enhance the fat solubility of the poison and greatly increase the time it acts within the animal's body.[4] However, as described above, the super-warfarins do not inhibit vitamin K and their effect is easily inhibited by vitamin K. Nevertheless, oral vitamin K may need to be given for times that may exceed a month, in order to counter the effect of second-generation VKAs that have very long residence times in the fat of animals and humans.
For a more complete list of coumarins used as pharmaceuticals and rodenticides, see the main article on 4-hydroxycoumarins.
-

Warfarin (Coumadin)
Other VKAs
Not all VKAs are coumarins. Examples include fluindione[5] and phenindione.[6]
Many of the non-coumarin VKAs are 1,3-indandione derivatives. All of these molecules share the same mechanism of action, and are inhibitors (antagonists) of vitamin K epoxide reductase, even though loosely called "vitamin K antagonists." The action of all of them may be antagonized by administration of vitamin K.
See also
- Vitamin K deficiency
- Category:Vitamin K antagonists
References
- ↑ Olmos V, López CM (2007). "Brodifacoum Poisoning with Toxicokinetic Data". Clinical Toxicology 45 (5): 487–9. doi:10.1080/15563650701354093. PMID 17503253.
- ↑ Schaefer C, Hannemann D, Meister R et al. (June 2006). "Vitamin K antagonists and pregnancy outcome. A multi-centre prospective study". Thromb. Haemost. 95 (6): 949–57. doi:10.1160/TH06-02-0108. PMID 16732373.
- ↑ Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G (June 2008). "Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition)". Chest 133 (6 Suppl): 160S–198S. doi:10.1378/chest.08-0670. PMID 18574265.
- ↑ Griminger P (July 1987). "Vitamin K antagonists: the first 50 years" (PDF). J. Nutr. 117 (7): 1325–9. PMID 3302140.
- ↑ Mentré F, Pousset F, Comets E et al. (January 1998). "Population pharmacokinetic-pharmacodynamic analysis of fluindione in patients". Clin. Pharmacol. Ther. 63 (1): 64–78. doi:10.1016/S0009-9236(98)90122-9. PMID 9465843.
- ↑ "Foreword: contemporary issues in the management and treatment of atrial fibrillation -- Agnelli 7 (2005): C3 -- European Heart Journal Supplements". Retrieved 2008-12-22.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||