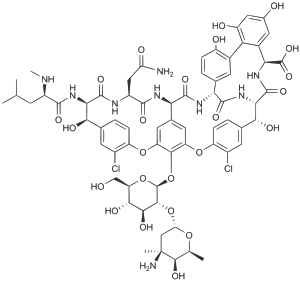
Vancomycin
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
| (1S,2R,18R,19R,22S,25R,28R,40S)- 48- {[(2S,3R,4S,5S,6R)- 3- {[(2S,4S,5S,6S)- 4- amino- 5- hydroxy- 4,6- dimethyloxan- 2- yl]oxy}- 4,5- dihydroxy- 6- (hydroxymethyl)oxan- 2- yl]oxy}- 22- (carbamoylmethyl)- 5,15- dichloro- 2,18,32,35,37- pentahydroxy- 19- [(2R)- 4- methyl- 2- (methylamino)pentanamido]- 20,23,26,42,44- pentaoxo- 7,13- dioxa- 21,24,27,41,43- pentaazaoctacyclo[26.14.2.23,6.214,17.18,12.129,33.010,25.034,39]pentaconta- 3,5,8(48),9,11,14,16,29(45),30,32,34,36,38,46,49- pentadecaene- 40- carboxylic acid | |
| Clinical data | |
| Trade names | Vancocin |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a604038 |
| Licence data | US FDA:link |
| |
| IV, oral | |
| Pharmacokinetic data | |
| Bioavailability | Negligible (oral) |
| Metabolism | Excreted unchanged |
| Half-life |
4–11 hours (adults) 6-10 days (adults, impaired renal function) |
| Excretion | Renal |
| Identifiers | |
|
1404-90-6 | |
| A07AA09 J01XA01 | |
| PubChem | CID 14969 |
| DrugBank |
DB00512 |
| ChemSpider |
14253 |
| UNII |
6Q205EH1VU |
| KEGG |
D00212 |
| ChEBI |
CHEBI:28001 |
| ChEMBL |
CHEMBL262777 |
| Chemical data | |
| Formula | C66H75Cl2N9O24 |
| 1449.3 g.mol−1 | |
|
SMILES
| |
| |
| | |

Vancomycin INN /væŋkɵˈmaɪsɨn/ is an antibiotic used to treat a number of bacterial infections. It is a member of the glycopeptide antibiotic class and is effective mostly against Gram-positive bacteria. Vancomycin was first isolated in 1953 at Eli Lilly & Co. from a soil sample collected by a missionary in the interior jungles of Borneo. It is a naturally occurring antibiotic made by the soil bacterium Amycolatopsis orientalis (formerly designated Nocardia orientalis). Chemically, vancomycin is a complex compound and an example of a comparatively rare halo-organic natural compound, containing two covalently bonded chlorine atoms (see green "balls" in ball-and-stick model, right).
The compound was industrially produced by fermentation and given the generic name vancomycin, derived from the term "vanquish". Clinically, vancomycin was used initially to treat infections caused by penicillin-resistant Staphylococcus aureus, a use which continued for many years because bacteria were relatively slow to acquire vancomycin resistance, even in experiments.
Currently, vancomycin is primarily used to treat serious infections caused by gram-positive bacteria which are known or suspected to be resistant to other antibiotics. The Infectious Disease Society of America recommends vancomycin as a first-line treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis caused by methicillin-resistant S. aureus.[1] Orally administered vancomycin is recommended as a treatment for intestinal infection with Clostridium difficile, a common side effect of treatment with broad-spectrum antibiotics.[2] Vancomycin is on the World Health Organization's List of Essential Medicines, a list of the most important medications needed in a basic health system.[3]
Medical uses
Vancomycin is indicated for the treatment of serious, life-threatening infections by gram-positive bacteria unresponsive to other antibiotics. In particular, vancomycin should not be used to treat methicillin-sensitive Staphylococcus aureus because it is inferior to penicillins such as nafcillin.[4][5]
The increasing emergence of vancomycin-resistant enterococci has resulted in the development of guidelines for use by the Centers for Disease Control Hospital Infection Control Practices Advisory Committee. These guidelines restrict use of vancomycin to the following indications:[6][7]
- Treatment of serious infections caused by susceptible organisms resistant to penicillins (methicillin-resistant S. aureus]] *MRSA) and multiresistant Staphylococcus epidermidis (MRSE)) or in individuals with serious allergy to penicillins
- Treatment of pseudomembranous colitis caused by Clostridium difficile; in particular, in cases of relapse or where the infection is unresponsive to metronidazole treatment (for this indication, vancomycin is given orally, rather than by its typical intravenous (IV) route)
- For treatment of infections caused by gram-positive microorganisms in patients with serious allergies to beta-lactam antimicrobials.[7]
- Antibacterial prophylaxis for endocarditis following certain procedures in penicillin-hypersensitive individuals at high risk[7]
- Surgical prophylaxis for major procedures involving implantation of prostheses in institutions with a high rate of MRSA or MRSE[7]
- Early in treatment as an empiric antibiotic for possible MRSA infection while waiting for culture identification of the infecting organism
Side effects
Serum vancomycin levels may be monitored in an effort to reduce side effects, although the value of such monitoring has been questioned.[8] Peak and trough levels are usually monitored, and, for research purposes, the area under the concentration curve is also sometimes used. Toxicity is best monitored by looking at trough values.[9]
Common adverse drug reactions (≥1% of patients) associated with IV vancomycin include: local pain, which may be severe, and thrombophlebitis.
Damage to the kidneys and to the hearing were a side effect of the early impure versions of vancomycin, and these were prominent in the clinical trials conducted in the mid-1950s.[10][11] Later trials using purer forms of vancomycin found nephrotoxicity is an infrequent adverse effect (0.1–1% of patients), but this is accentuated in the presence of aminoglycosides.[12]
Rare adverse effects (<0.1% of patients) include: anaphylaxis, toxic epidermal necrolysis, erythema multiforme, red man syndrome, superinfection, thrombocytopenia, neutropenia, leukopenia, tinnitus, and dizziness and/or ototoxicity
Vancomycin can induce platelet-reactive antibodies in the patient, leading to severe thrombocytopenia and bleeding with florid petechial hemorrhages, ecchymoses, and wet purpura.[13]
Vancomycin has traditionally been considered a nephrotoxic and ototoxic drug, based on observations by early investigators of elevated serum levels in renally impaired patients who had experienced ototoxicity, and subsequently through case reports in the medical literature. However, as the use of vancomycin increased with the spread of MRSA beginning in the 1970s, the previously reported rates of toxicity were recognized as not being observed. This was attributed to the removal of the impurities present in the earlier formulation of the drug, although those impurities were not specifically tested for toxicity.[10]
Nephrotoxicity
Subsequent reviews of accumulated case reports of vancomycin-related nephrotoxicity found many of the patients had also received other known nephrotoxins, in particular, aminoglycosides. Most of the rest had other confounding factors, or insufficient data regarding the possibility of such, that prohibited the clear association of vancomycin with the observed renal dysfunction.
In 1994, the use of vancomycin monotherapy was clearly documented in only three of 82 available cases in the literature.[14] Prospective and retrospective studies attempting to evaluate the incidence of vancomycin-related nephrotoxicity have largely been methodologically flawed and have produced variable results. The most methodologically sound investigations indicate the actual incidence of vancomycin-induced nephrotoxicity is around 5–7%. To put this into context, similar rates of renal dysfunction have been reported for cefamandole and benzylpenicillin, two reputedly non-nephrotoxic antibiotics.
In addition, evidence to relate nephrotoxicity to vancomycin serum levels is inconsistent. Some studies have indicated an increased rate of nephrotoxicity when trough levels exceed 10 µg/ml, but others have not reproduced these results. Nephrotoxicity has also been observed with concentrations within the "therapeutic" range, as well. In essence, the reputation of vancomycin as a nephrotoxin is overstated, and it has not been demonstrated that maintaining vancomycin serum levels within certain ranges will prevent its nephrotoxic effects, when they do occur.
Ototoxicity
Attempts to establish rates of vancomycin-induced ototoxicity are even more difficult due to the scarcity of quality evidence. The current consensus is that clearly related cases of vancomycin ototoxicity are rare. The association between vancomycin serum levels and ototoxicity is also uncertain. While cases of ototoxicity have been reported in patients whose vancomycin serum level exceeded 80 µg/ml, cases have been reported in patients with therapeutic levels, as well. Thus, whether therapeutic drug monitoring of vancomycin for the purpose of maintaining "therapeutic" levels will prevent ototoxicity also remains unproven.
Interactions with other nephrotoxins
Another area of controversy and uncertainty concerns the question of whether, and, if so, to what extent, vancomycin increases the toxicity of other nephrotoxins. Clinical studies have yielded variable results, but animal models indicate some increased nephrotoxic effect probably occurs when vancomycin is added to nephrotoxins such as aminoglycosides. However, a dose- or serum level-effect relationship has not been established.
Dosing considerations
Intravenous vs oral administration
Vancomycin must be given intravenously (IV) for systemic therapy, since it is not absorbed from the intestine. It is a large hydrophilic molecule that partitions poorly across the gastrointestinal mucosa. Due to short half-life, it is often injected twice-daily.[15]
The only approved indication for oral vancomycin therapy is in the treatment of pseudomembranous colitis, where it must be given orally to reach the site of infection in the colon. Following oral administration, the fecal concentration of vancomycin is around 500 µg/ml[16] (sensitive strains of C. difficile have a mean inhibitory concentration of ≤2 µg/ml[17])
Inhaled vancomycin has also been used (off-label), via nebulizer, for treatment of various infections of the upper and lower respiratory tract.
The caustic nature of vancomycin makes IV therapy using peripheral lines a risk for thrombophlebitis. Ideally, central lines or infusion ports should be used.[18]
Red man syndrome
Vancomycin is recommended to be administered in a dilute solution slowly, over at least 60 minutes (maximum rate of 10 mg/minute for doses >500 mg)[6] due to the high incidence of pain and thrombophlebitis and to avoid an infusion reaction known as the "red man syndrome" or "red neck syndrome". This syndrome, usually appearing within 4–10 min after the commencement or soon after the completion of an infusion, is characterized by flushing and/or an erythematous rash that affects the face, neck, and upper torso. These findings are due to nonspecific mast cell degranulation and are not an IgE-mediated allergic reaction. Less frequently, hypotension and angioedema may also occur. Symptoms may be treated or prevented with antihistamines, including diphenhydramine, and are less likely to occur with slow infusion.[19][20]:120–1
Therapeutic drug monitoring
Plasma level monitoring of vancomycin is necessary due to the drug's biexponential distribution, intermediate hydrophilicity, and potential for ototoxicity and nephrotoxicity, especially in populations with poor renal function and/or increased propensity to bacterial infection. Vancomycin activity is considered to be time-dependent; that is, antimicrobial activity depends on the duration that the serum drug concentration exceeds the minimum inhibitory concentration of the target organism. Thus, peak serum levels have not been shown to correlate with efficacy or toxicity; indeed, concentration monitoring is unnecessary in most cases. Circumstances in which therapeutic drug monitoring is warranted include: patients receiving concomitant aminoglycoside therapy, patients with (potentially) altered pharmacokinetic parameters, patients on haemodialysis, patients administered high-dose or prolonged treatment, and patients with impaired renal function. In such cases, trough concentrations are measured.[6][14][21][22]
Target ranges for serum vancomycin concentrations have changed over the years. Early authors suggested peak levels of 30–40 mg/l and trough levels of 5–10 mg/l,[23] but current recommendations are that peak levels need not be measured and that trough levels of 10-15 or 15–20 mg/l, depending on the nature of the infection and the specific needs of the patient, may be appropriate.[24][25]
Biosynthesis

Vancomycin biosynthesis occurs via different nonribosomal protein synthases (NRPSs).[26] The enzymes determine the amino acid sequence during its assembly through its 7 modules. Before vancomycin is assembled through NRPS, the amino acids are first modified. L-tyrosine is modified to become the β-hydroxychlorotyrosine (β-hTyr) and 4-hydroxyphenylglycine (HPG) residues. On the other hand, acetate is used to derive the 3,5 dihydroxyphenylglycine ring (3,5-DPG).[27]

Nonribosomal peptide synthesis occurs through distinct modules that can load and extend the protein by one amino acid through the amide bond formation at the contact sites of the activating domains.[28] Each module typically consists of an adenylation (A) domain, a peptidyl carrier protein (PCP) domain, and a condensation (C) or elongation domain. In the A domain, the specific amino acid is activated by converting into an aminoacyl adenylate enzyme complex attached to a 4'phosphopantetheine cofactor by thioesterification[29][30] The complex is then transferred to the PCP domain with the expulsion of AMP. The PCP domain uses the attached 4'-phosphopantethein prosthetic group to load the growing peptide chain and their precursors.[31] The organization of the modules necessary to biosynthesize Vancomycin is shown in Figure 1. In the biosynthesis of Vancomycin, additional modification domains are present, such as the epimerization (E) domain, which isomerizes the amino acid from one stereochemistry to another, and a thioesterase domain (TE) is used as a catalyst for cyclization and releases of the molecule via a thioesterase scission.

A set of multienzymes (peptide synthase CepA, CepB, and CepC) are responsible for assembling the heptapeptide. (Figure 2). The organization of CepA, CepB, and Cep C closely resembles other peptide synthases such as those for surfactin (SrfA1, SrfA2, and SrfA3) and gramicidin (GrsA and GrsB).[28] Each peptide synthase activates codes for various amino acids to activate each domain. CepA codes for modules 1, 2, and 3. CepB codes for modules 4, 5, and 6, and CepC codes for module 7. The three peptide synthases are located at the start of the region of the bacterial genome linked with antibiotic biosynthesis, and spans 27 kb.[28]
After the linear heptapeptide molecule is synthesized, vancomycin has to undergo further modifications, such as oxidative cross-linking and glycosylation, in trans by distinct enzymes, referred to as tailoring enzymes, to become biologically active (Figure 3). To convert the linear heptapeptide, eight enzymes, open reading frames (ORFs) 7, 8, 9, 10, 11, 14, 18, 20, and 21 are used. The enzymes ORF 7, 8, 9, and 20 are P450 enzymes. ORF 10 and 18 show to nonheme haloperoxidases. And ORF 9 and 14 are identified as putative hydroxylation enzymes.[32] With the help of these enzymes, β-hydroxyl groups are introduced onto tyrosine residues 2 and 6, and coupling occurs for rings 5 and 7, rings 4 and 6, and rings 4 and 2. In addition, a haloperoxidase is used to attach the chlorine atoms onto rings 2 and 6 via an oxidative process.[28] Some of the glycosyltransferases capable of glycosylating vancomycin and related nonribosomal peptides display notable permissivity and have been employed for generating libraries of differentially glycosylated analogs through a process known as glycorandomization.[33][34][35]
Spectrum of susceptibility
Vancomycin is considered a last resort medication for the treatment of septicemia and lower respiratory tract, skin, and bone infections caused by gram-positive bacteria. The MIC susceptibility data for a few medically significant bacteria are:
- Staphylococus aureus: 0.25 - 4.0 μg/ml
- Staphylococcus aureus (methicillin resistant or MRSA): 1 - 138 μg/ml
- Staphylococcus epidermidis: ≤0.12 - 6.25 μg/ml
Pharmacology and chemistry
Vancomycin is a branched tricyclic glycosylated nonribosomal peptide produced by the Actinobacteria species Amycolatopsis orientalis (formerly designated Nocardia orientalis).
Vancomycin exhibits atropisomerism — it has multiple chemically distinct rotamers owing to the rotational restriction of some of the bonds. The form present in the drug is the thermodynamically more stable conformer, so has more potent activity.
Mechanism of action
Vancomycin acts by inhibiting proper cell wall synthesis in gram-positive bacteria. Due to the different mechanism by which gram-negative bacteria produce their cell walls and the various factors related to entering the outer membrane of gram-negative organisms, vancomycin is not active against gram-negative bacteria (except some nongonococcal species of Neisseria).
The large hydrophilic molecule is able to form hydrogen bond interactions with the terminal D-alanyl-D-alanine moieties of the NAM/NAG-peptides. Under normal circumstances, this is a five-point interaction. This binding of vancomycin to the D-Ala-D-Ala prevents cell wall synthesis of the long polymers of N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG) that form the backbone strands of the bacterial cell wall, and it prevents the backbone polymers that do manage to form from cross-linking with each other.[37]

Mechanism of vancomycin action and resistance: This diagram shows only one of two ways vancomycin acts against bacteria (inhibition of cell wall cross-linking) and only one of many ways that bacteria can become resistant to it.
- Vancomycin is added to the bacterial environment while it is trying to synthesize new cell wall. Here, the cell wall strands have been synthesized, but not yet cross-linked.
- Vancomycin recognizes and binds to the two D-ala residues on the end of the peptide chains. However, in resistant bacteria, the last D-ala residue has been replaced by a D-lactate, so vancomycin cannot bind.
- In resistant bacteria, cross-links are successfully formed. However, in the nonresistant bacteria, the vancomycin bound to the peptide chains prevents them from interacting properly with the cell wall cross-linking enzyme.
- In the resistant bacteria, stable cross links are formed. In the sensitive bacteria, cross-links cannot be formed and the cell wall falls apart.
Usage in plant tissue culture
Vancomycin is one of the few antibiotics used in plant tissue culture to eliminate gram-positive bacteria infection. It has relatively low toxicity to plants.[38][39]
Antibiotic resistance
Intrinsic resistance
A few gram-positive bacteria are intrinsically resistant to vancomycin: Leuconostoc and Pediococcus species, but these organisms rarely cause diseases in humans.[40] Most Lactobacillus species are also intrinsically resistant to vancomycin,[40] with the exception of L. acidophilus and L. delbruekii, which are sensitive.[41] Other gram-positive bacteria with intrinsic resistance to vancomycin include Erysipelothrix rhusiopathiae, Weissella confusa, and Clostridium innocuum.[42][43][44]
Most gram-negative bacteria are intrinsically resistant to vancomycin because their outer membrane is impermeable to large glycopeptide molecules[45] (with the exception of some non-gonococcal Neisseria species).[46]
Acquired resistance
Evolution of microbial resistance to vancomycin is a growing problem, in particular, within healthcare facilities such as hospitals. While newer alternatives to vancomycin exist, such as linezolid (2000) and daptomycin (2003), the widespread use of vancomycin makes resistance to the drug a significant worry, especially for individual patients if resistant infections are not quickly identified and the patient continues the ineffective treatment. Vancomycin-resistant Enterococcus emerged in 1987. Vancomycin resistance evolved in more common pathogenic organisms during the 1990s and 2000s, including vancomycin-intermediate Staphylococcus aureus (VISA) and vancomycin-resistant Staphylococcus aureus (VRSA).[47][48] Agricultural use of avoparcin, another similar glycopeptide antibiotic, may have contributed to the evolution of vancomycin-resistant organisms.[49][50][51][52]
One mechanism of resistance to vancomycin involves the alteration to the terminal amino acid residues of the NAM/NAG-peptide subunits, under normal conditions, D-alanyl-D-alanine, to which vancomycin binds. The D-alanyl-D-lactate variation results in the loss of one hydrogen-bonding interaction (4, as opposed to 5 for D-alanyl-D-alanine) possible between vancomycin and the peptide. This loss of just one point of interaction results in a 1000-fold decrease in affinity. The D-alanyl-D-serine variation causes a six-fold loss of affinity between vancomycin and the peptide, likely due to steric hindrance.[53]
In enterococci, this modification appears to be due to the expression of an enzyme that alters the terminal residue. Three main resistance variants have been characterised to date among resistant Enterococcus faecium and E. faecalis populations:
- VanA - Enterococcal resistance to vancomycin and teicoplanin; inducible on exposure to these agents
- VanB - lower-level enterococcal resistance; inducible by vancomycin, but strains may remain susceptible to teicoplanin
- VanC - least clinically important; enterococci resistant only to vancomycin; constitutive resistance
2011: A variant of vancomycin has been tested at the Scripps Research Institute that binds to the resistant D-lactic acid variation in vancomycin-resistant bacterial cell walls, and also binds well to the original target (vancomycin-susceptible bacteria), and thus reinstates potent antimicrobial activity.[54]
History
Vancomycin was first isolated in 1953 by Edmund Kornfeld (working at Eli Lilly) from a soil sample collected from the interior jungles of Borneo by a missionary.[55] The organism that produced it was eventually named Amycolatopsis orientalis.[10] The original indication for vancomycin was for the treatment of penicillin-resistant Staphylococcus aureus.[10][11]
The compound was initially called compound 05865, but was eventually given the generic name vancomycin, derived from the term "vanquish".[10] One advantage that was quickly apparent is that staphylococci did not develop significant resistance despite serial passage in culture media containing vancomycin. The rapid development of penicillin resistance by staphylococci led to the compound's being fast-tracked for approval by the Food and Drug Administration in 1958. Eli Lilly first marketed vancomycin hydrochloride under the trade name Vancocin[11]
Vancomycin never became the first-line treatment for S. aureus for several reasons:
- It possesses poor oral bioavailability; it must be given intravenously for most infections.
- β-Lactamase-resistant semisynthetic penicillins such as methicillin (and its successors, nafcillin and cloxacillin) were subsequently developed, which have better activity against non-MRSA staphylococci.
- Early trials used early impure forms of vancomycin ("Mississippi mud"), which were found to be toxic to the ears and to the kidneys;[56] these findings led to vancomycin's being relegated to the position of a drug of last resort.[11]
In 2004, Eli Lilly licensed Vancocin to ViroPharma in the U.S., Flynn Pharma in the UK, and Aspen Pharmacare in Australia. The patent expired in the early 1980s; the FDA authorized the sale of several generic versions in the USA, including from manufacturers Bioniche Pharma, Baxter Healthcare, Sandoz, Akorn Strides, and Hospira.[57]
See also
- Antibiotic resistance
- Drug of last resort
- Drug resistance
- Glycorandomization
- Teixobactin
References
- ↑ "www.idsociety.org" (PDF).
- ↑ "www.idsociety.org" (PDF).
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ Small PM, Chambers HF (1990). "Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users". Antimicrob Agents Chemother 34 (6): 1227–31. doi:10.1128/AAC.34.6.1227. PMC 171789. PMID 2393284.
- ↑ Gonzalez C, Rubio M, Romero-Vivas J, Gonzalez M, Picazo JJ (1999). "Bacteremic pneumonia due to Staphylococcus aureus: a comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms". Clin Infect Dis 29 (5): 1171–7. doi:10.1086/313440. PMID 10524959.
- ↑ 6.0 6.1 6.2 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ↑ 7.0 7.1 7.2 7.3 "Recommendations for Preventing the Spread of Vancomycin Resistance Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC)". MMWR Recomm Rep 44 (RR-12): 1–13. September 1995. PMID 7565541.
- ↑ Cantú TG, Yamanaka-Yuen NA, Lietman PS (1994). "Serum vancomycin concentrations: reappraisal of their clinical value". Clin Infect Dis 18 (4): 533–43. doi:10.1093/clinids/18.4.533. PMID 8038306.
- ↑ Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL (2009). "Relationship between initial vancomycin concentration‐time profile and nephrotoxicity (toxic to the kidneys) among hospitalized patients". Clin Infect Dis 49 (4): 507–514. doi:10.1086/600884. PMID 19586413.
- ↑ 10.0 10.1 10.2 10.3 10.4 Levine, D. (2006). "Vancomycin: A History". Clin Infect Dis 42: S5–S12. doi:10.1086/491709. PMID 16323120.
- ↑ 11.0 11.1 11.2 11.3 Moellering, RC Jr. (January 2006). "Vancomycin: a 50-year reassessment". Clin Infect Dis. 42 Suppl 1: S3–4. doi:10.1086/491708. PMID 16323117.
- ↑ Farber BF, Moellering RC Jr. (1983). "Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981". Antimicrob Agents Chemother 23 (1): 138–41. doi:10.1128/AAC.23.1.138. PMC 184631. PMID 6219616.
- ↑ Drygalski A, Curtis BR (2007). "Vancomycin-Induced Immune Thrombocytopenia". N Engl J Med 356 (9): 904–10. doi:10.1056/NEJMoa065066. PMID 17329697.
- ↑ 14.0 14.1 Cantú, TG; Yamanaka-Yuen, NA; Lietman, PS (1994). "Serum vancomycin concentrations: reappraisal of their clinical value". Clinical Infectious Diseases 18 (4): 533–43. doi:10.1093/clinids/18.4.533. PMID 8038306.
- ↑ Van Bambeke F (August 2006). "Glycopeptides and glycodepsipeptides in clinical development: a comparative review of their antibacterial spectrum, pharmacokinetics and clinical efficacy". Current Opinion in Investigational Drugs 7 (8): 740–9. PMID 16955686.
- ↑ Edlund C, Barkholt L, Olsson-Liljequist B, Nord CE (September 1997). "Effect of vancomycin on intestinal flora of patients who previously received antimicrobial therapy". Clinical Infectious Diseases 25 (3): 729–32. doi:10.1086/513755. PMID 9314469.
- ↑ Peláez T, Alcalá L, Alonso R et al. (2002). "Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin". Antimicrob Agents Chemother 46 (6): 1647–1650. doi:10.1128/AAC.46.6.1647-1650.2002.
- ↑ Choosing the Right Intravenous Catheter
- ↑ Sivagnanam S, Deleu D (April 2003). "Red man syndrome". Critical Care 7 (2): 119–20. doi:10.1186/cc1871. PMC 270616. PMID 12720556.
- ↑ James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology. (10th ed.). Saunders. ISBN 0-7216-2921-0.
- ↑ Moellering Jr, RC (1994). "Monitoring serum vancomycin levels: climbing the mountain because it is there?". Clinical Infectious Diseases 18 (4): 544–6. doi:10.1093/clinids/18.4.544. PMID 8038307.
- ↑ Karam, CM; McKinnon, PS; Neuhauser, MM; Rybak, MJ (1999). "Outcome assessment of minimizing vancomycin monitoring and dosing adjustments". Pharmacotherapy 19 (3): 257–66. doi:10.1592/phco.19.4.257.30933. PMID 10221365.
- ↑ Geraci J (1977). "Vancomycin". Mayo Clin Proc 52 (10): 631–4. PMID 909314.
- ↑ Rybak M, Lomaestro B, Rotschafer JC et al. (January 2009). "Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists". American Journal of Health-system Pharmacy 66 (1): 82–98. doi:10.2146/ajhp080434. PMID 19106348.
- ↑ Thomson AH, Staatz CE, Tobin CM, Gall M, Lovering AM (May 2009). "Development and evaluation of vancomycin dosage guidelines designed to achieve new target concentrations". The Journal of Antimicrobial Chemotherapy 63 (5): 1050–7. doi:10.1093/jac/dkp085. PMID 19299472.
- ↑ Samel SA, Marahiel MA, Essen LO (May 2008). "How to tailor non-ribosomal peptide products-new clues about the structures and mechanisms of modifying enzymes". Mol Biosyst 4 (5): 387–93. doi:10.1039/b717538h. PMID 18414736.
- ↑ Dewick, Paul M. (2002). Medicinal natural products: a biosynthetic approach. New York: Wiley. ISBN 0-471-49641-3.
- ↑ 28.0 28.1 28.2 28.3 van Wageningen AM, Kirkpatrick PN, Williams DH et al. (March 1998). "Sequencing and analysis of genes involved in the biosynthesis of a vancomycin group antibiotic". Chem. Biol. 5 (3): 155–62. doi:10.1016/S1074-5521(98)90060-6. PMID 9545426.
- ↑ Schlumbohm W, Stein T, Ullrich C et al. (December 1991). "An active serine is involved in covalent substrate amino acid binding at each reaction center of gramicidin S synthetase". J. Biol. Chem. 266 (34): 23135–41. PMID 1744112.
- ↑ Stein T, Vater J, Kruft V et al. (June 1996). "The multiple carrier model of nonribosomal peptide biosynthesis at modular multienzymatic templates". The Journal of Biological Chemistry 271 (26): 15428–35. doi:10.1074/jbc.271.26.15428. PMID 8663196.
- ↑ Kohli RM, Walsh CT, Burkart MD (August 2002). "Biomimetic synthesis and optimization of cyclic peptide antibiotics". Nature 418 (6898): 658–61. doi:10.1038/nature00907. PMID 12167866.
- ↑ Solenberg PJ, Matsushima P, Stack DR, Wilkie SC, Thompson RC, Baltz RH (March 1997). "Production of hybrid glycopeptide antibiotics in vitro and in Streptomyces toyocaensis". Chemistry & Biology 4 (3): 195–202. doi:10.1016/S1074-5521(97)90288-X. PMID 9115410.
- ↑ Fu X, Albermann C, Jiang J, Liao J, Zhang C, Thorson JS (December 2003). "Antibiotic optimization via in vitro glycorandomization". Nature Biotechnology 21 (12): 1467–9. doi:10.1038/nbt909. PMID 14608364.
- ↑ Fu X, Albermann C, Zhang C, Thorson JS (April 2005). "Diversifying vancomycin via chemoenzymatic strategies". Organic Letters 7 (8): 1513–5. doi:10.1021/ol0501626. PMID 15816740.
- ↑ Peltier-Pain P, Marchillo K, Zhou M, Andes DR, Thorson JS (October 2012). "Natural product disaccharide engineering through tandem glycosyltransferase catalysis reversibility and neoglycosylation". Organic Letters 14 (19): 5086–9. doi:10.1021/ol3023374. PMC 3489467. PMID 22984807.
- ↑ http://antibiotics.toku-e.com/antimicrobial_1182_1.html[]
- ↑ Clinical Pharmacology
- ↑ vancomcin for plant cell culture
- ↑ Pazuki, A; Asghari, J; Sohani, M; Pessarakli, M & Aflaki, F (2014). "Effects of Some Organic Nitrogen Sources and Antibiotics on Callus Growth of Indica Rice Cultivars" (PDF). Journal of Plant Nutrition 00 (0): 00–00. doi:10.1080/01904167.2014.983118. Retrieved February 17, 2014.
- ↑ 40.0 40.1 Swenson JM, Facklam RR, Thornsberry C (April 1990). "Antimicrobial susceptibility of vancomycin-resistant Leuconostoc, Pediococcus, and Lactobacillus species". Antimicrob Agents Chemother 34 (4): 543–9. doi:10.1128/AAC.34.4.543. PMC 171641. PMID 2344161.
- ↑ Hamilton-Miller JM, Shah S (February 1998). "Vancomycin susceptibility as an aid to the identification of lactobacilli". Lett Appl Microbiol 26 (2): 153–4. doi:10.1046/j.1472-765X.1998.00297.x. PMID 9569701.
- ↑ Romney, M; Cheung, S; Montessori, V (2001). "Erysipelothrix rhusiopathiae endocarditis and presumed osteomyelitis". The Canadian Journal of Infectious Diseases 12 (4): 254–256. PMC 2094827. PMID 18159347.
- ↑ David V, Bozdogan B, Mainardi JL, Legrand R, Gutmann L, Leclercq R (June 2004). "Mechanism of intrinsic resistance to vancomycin in Clostridium innocuum NCIB 10674". Journal of Bacteriology 186 (11): 3415–22. doi:10.1128/JB.186.11.3415-3422.2004. PMC 415764. PMID 15150227.
- ↑ Kumar A, Augustine D, Sudhindran S et al. (October 2011). "Weissella confusa: a rare cause of vancomycin-resistant Gram-positive bacteraemia". Journal of Medical Microbiology 60 (Pt 10): 1539–41. doi:10.1099/jmm.0.027169-0. PMID 21596906.
- ↑ Quintiliani R Jr, Courvalin P (1995). "Mechanisms of Resistance to Antimicrobial Agents". In Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH. Manual of Clinical Microbiology (6th ed.). Washington DC: ASM Press. p. 1319. ISBN 1-55581-086-1.
- ↑ Geraci JE, Wilson WR (1981). "Vancomycin therapy for infective enocarditis". Rev Infect Dis. 3 suppl: S250–8. PMID 7342289.
- ↑ Smith, TL; Pearson, ML; Wilcox, KR; Cruz, C; Lancaster, MV; Robinson-Dunn, B; Tenover, FC; Zervos, MJ; Band, JD (February 1999). "Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group". The New England Journal of Medicine (PDF) 340 (7): 493–501. doi:10.1056/NEJM199902183400701. PMID 10021469.
- ↑ McDonald, LC; Killgore, GE; Thompson, A; Owens Jr, RC; Kazakova, SV; Sambol, SP; Johnson, S; Gerding, DN (December 2005). "An epidemic, toxin gene-variant strain of Clostridium difficile". The New England Journal of Medicine (PDF) 353 (23): 2433–41. doi:10.1056/NEJMoa051590. PMID 16322603.
- ↑ Acar, J.; Casewell, M.; Freeman, J.; Friis, C.; Goossens, H. (2000). "Avoparcin and virginiamycin as animal growth promoters: A plea for science in decision-making". Clinical Microbiology and Infection 6 (9): 477–82. doi:10.1046/j.1469-0691.2000.00128.x. PMID 11168181.
- ↑ Bager, F; Madsen, M; Christensen, J; Aarestrup, F.M (1997). "Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms". Preventive Veterinary Medicine 31 (1–2): 95–112. doi:10.1016/S0167-5877(96)01119-1. PMID 9234429.
- ↑ Peter J Collignon (1999). "Vancomycin-resistant enterococci and use of avoparcin in animal feed: is there a link?". Med J Aust 171 (3): 144–146. PMID 10474607.
- ↑ Lauderdale, TL; Shiau, YR; Wang, HY; Lai, JF; Huang, IW; Chen, PC; Chen, HY; Lai, SS; Liu, YF (2007). "Effect of banning vancomycin analogue avoparcin on vancomycin-resistant enterococci in chicken farms in Taiwan". Environmental microbiology 9 (3): 819–23. doi:10.1111/j.1462-2920.2006.01189.x. PMID 17298380.
- ↑ Pootoolal J, Neu J, Wright GD (2002). "Glycopeptide antibiotic resistance". Annu Rev Pharmacol Toxicol 42: 381–408. doi:10.1146/annurev.pharmtox.42.091601.142813. PMID 11807177.
- ↑ Xie, J; Pierce, JG; James, RC; Okano, A; Boger, DL (September 2011). "A Redesigned Vancomycin Engineered for Dual d-Ala-d-Ala and d-Ala-d-Lac Binding Exhibits Potent Antimicrobial Activity Against Vancomycin-Resistant Bacteria". J. Am. Chem. Soc. 133 (35): 13946–9. doi:10.1021/ja207142h. PMC 3164945. PMID 21823662.
- ↑ Shnayerson, Michael; Plotkin, Mark (2003). The Killers Within: The Deadly Rise of Drug-Resistant Bacteria. Back Bay Books. ISBN 978-0-316-73566-7.
- ↑ Griffith RS. (1981). "Introduction to vancomycin". Rev Infect Dis 3: S200–S204. PMID 7043707.
- ↑ Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations
| ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||