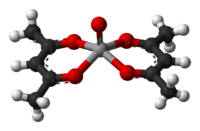
Vanadyl acetylacetonate
 | |
| Names | |
|---|---|
| IUPAC name
oxobis(2,4-pentanedionato)vanadium(IV) | |
| Other names
VO(acac)2, VO(pd)2 | |
| Identifiers | |
| 3153-26-2 | |
| PubChem | 16217092 |
| Properties | |
| C10H14O5V | |
| Molar mass | 265.157 g/mol |
| Appearance | blue-green |
| Density | 1.50 g/cm3 |
| Melting point | 258 °C (496 °F; 531 K) |
| Boiling point | 174 °C (345 °F; 447 K) (at 0.2 torr) |
| CHCl3, CH2Cl2, Benzene, CH3OH, CH3CH2OH | |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Vanadyl acetylacetonate is the chemical compound with the formula VO(C5H7O2)2. This blue-green coordination complex consists of the vanadyl group, VO2+, bound to two acetylacetonate anions, acac−. Like other charge-neutral acetylacetonates, this complex is soluble in organic solvents.
Synthesis
The complex is prepared from vanadium(IV), e.g. vanadyl sulfate, or vanadium(V) precursors, such as vanadium pentoxide.[1] The equation for the redox reaction starting from the pentoxide can be described by this approximate equation:
- 2 V2O5 + 9 C5H8O2 → 4 VO(C5H7O2)2 + (CH3CO)2CO + 5 H2O
The compound is recrystallized from chloroform.
Structure and properties
The complex has a square pyramidal structure with a short V=O bond. This d1 compound is paramagnetic. Its optical spectrum exhibits two transitions. It is a weak Lewis acid, forming adducts with pyridine and methylamine.[1]
Applications
It is used in organic chemistry as a reagent in the epoxidation of allylic alcohols in combination with tert-butyl hydroperoxide (TBHP). The VO(acac)2-TBHP system exclusively epoxidizes geraniol at the allylic alcohol position. For comparison, another epoxidizing agent m-CPBA, reacts with both groups, creating the products in a two to one ratio, favoring the allylic position away from the alcohol. TBHP oxidizes VO(acac)2 to a vanadium(V) species which coordinates the alcohol of the substrate and the hydroperoxide.[2][3]
Biomedical aspects
Vanadyl(acac) exhibits insulin mimetic properties, in that in can stimulate the phosphorylation of protein kinase B (PKB/Akt) and glycogen synthase kinase-3 (GSK3).[4] It has also been shown inhibit tyrosine phosphatase(PTPase), PTPases such as PTP1B, which dephosphorylates insulin receptor beta subunit, thus increasing its phosphorylation, allowing for a prolonged activation of IRS-1, PKB, and GSK3, allowing them to exert their anti-diabetic properties.[4]
External links
References
- ↑ 1.0 1.1 Richard A. Rowe, Mark M. Jones (1957). "Vanadium(IV) Oxy(acetylacetonate)". Inorg. Synth. Inorganic Syntheses 5: 113–116. doi:10.1002/9780470132364.ch30. ISBN 978-0-470-13236-4.
- ↑ Takashi Itoh, Koichiro Jitsukawa, Kiyotomi Kaneda, Shiichiro Teranishi (1979). "Vanadium-catalyzed epoxidation of cyclic allylic alcohols. Stereoselectivity and stereocontrol mechanism". J. Am. Chem. Soc. 101 (1): 159–169. doi:10.1021/ja00495a027.
- ↑ Bryant E. Rossiter, Hsyueh-Liang Wu, Toshikazu Hirao "Vanadyl Bis(acetylacetonate)" in Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons, 2007. doi:10.1002/047084289X.rv003m.pub2. Article Online Posting Date: March 15, 2007
- ↑ 4.0 4.1 Mohamad Z. Mehdi and Ashok K. Srivastava (2005). "Organo-vanadium compounds are potent activators of the protein kinase B signaling pathway and protein tyrosine phosphorylation: Mechanism of insulinomimesis". ABB 440 (2): 158–164. doi:10.1016/j.abb.2005.06.008. PMID 16055077.