VPS25
| Vacuolar protein sorting 25 homolog (S. cerevisiae) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
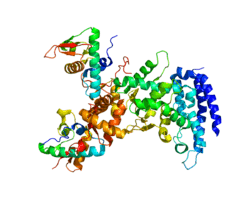 Rendering based on PDB 2ZME. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | VPS25 ; DERP9; EAP20; FAP20 | ||||||||||||
| External IDs | OMIM: 610907 MGI: 106354 HomoloGene: 6303 GeneCards: VPS25 Gene | ||||||||||||
| |||||||||||||
| RNA expression pattern | |||||||||||||
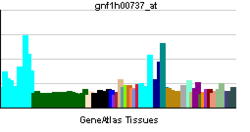 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | 84313 | 28084 | |||||||||||
| Ensembl | ENSG00000131475 | ENSMUSG00000078656 | |||||||||||
| UniProt | Q9BRG1 | Q9CQ80 | |||||||||||
| RefSeq (mRNA) | NM_032353 | NM_001284411 | |||||||||||
| RefSeq (protein) | NP_115729 | NP_001271340 | |||||||||||
| Location (UCSC) | Chr 17: 40.93 – 40.93 Mb | Chr 11: 101.25 – 101.26 Mb | |||||||||||
| PubMed search | |||||||||||||
| ESCRT-II complex subunit | |||||||||
|---|---|---|---|---|---|---|---|---|---|
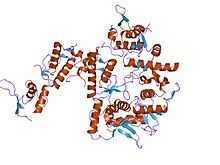 crystal structure of subunit vps25 of the endosomal trafficking complex escrt-ii | |||||||||
| Identifiers | |||||||||
| Symbol | ESCRT-II | ||||||||
| Pfam | PF05871 | ||||||||
| InterPro | IPR008570 | ||||||||
| |||||||||
Vacuolar protein-sorting-associated protein 25 is a protein that in humans is encoded by the VPS25 gene.[1][2]
It is a component of the endosome-associated complex ESCRT-II (Endosomal Sorting Complexes Required for Transport protein II). ESCRT (ESCRT-I, -II, -III) complexes orchestrate efficient sorting of ubiquitinated transmembrane receptors to lysosomes via multivesicular bodies (MVBs).[3] ESCRT-II recruits the transport machinery for protein sorting at MVB.[4] In addition, the human ESCRT-II has been shown to form a complex with RNA polymerase II elongation factor ELL in order to exert transcriptional control activity. ESCRT-II transiently associates with the endosomal membrane and thereby initiates the formation of ESCRT-III, a membrane-associated protein complex that functions immediately downstream of ESCRT-II during sorting of MVB cargo. ESCRT-II in turn functions downstream of ESCRT-I, a protein complex that binds to ubiquitinated endosomal cargo.[5]
ESCRT-II is a trilobal complex composed of two copies of vps25, one copy of vps22 and the C-terminal region of vps36. The crystal structure of vps25 revealed two winged-helix domains, the N-terminal domain of vps25 interacting with vps22 and vps36.[6]
References
- ↑ Yorikawa C, Shibata H, Waguri S, Hatta K, Horii M, Katoh K, Kobayashi T, Uchiyama Y, Maki M (Mar 2005). "Human CHMP6, a myristoylated ESCRT-III protein, interacts directly with an ESCRT-II component EAP20 and regulates endosomal cargo sorting". Biochem J 387 (Pt 1): 17–26. doi:10.1042/BJ20041227. PMC 1134928. PMID 15511219.
- ↑ "Entrez Gene: VPS25 vacuolar protein sorting 25 homolog (S. cerevisiae)".
- ↑ Gill DJ, Teo H, Sun J, Perisic O, Veprintsev DB, Emr SD, Williams RL (January 2007). "Structural insight into the ESCRT-I/-II link and its role in MVB trafficking". EMBO J. 26 (2): 600–12. doi:10.1038/sj.emboj.7601501. PMC 1783442. PMID 17215868.
- ↑ Teo H, Perisic O, Gonzalez B, Williams RL (October 2004). "ESCRT-II, an endosome-associated complex required for protein sorting: crystal structure and interactions with ESCRT-III and membranes". Dev. Cell 7 (4): 559–69. doi:10.1016/j.devcel.2004.09.003. PMID 15469844.
- ↑ Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD (August 2002). "Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body". Dev. Cell 3 (2): 283–9. doi:10.1016/S1534-5807(02)00219-8. PMID 12194858.
- ↑ Wernimont AK, Weissenhorn W (December 2004). "Crystal structure of subunit VPS25 of the endosomal trafficking complex ESCRT-II". BMC Struct. Biol. 4 (1): 10. doi:10.1186/1472-6807-4-10. PMC 539351. PMID 15579210.
Further reading
- Kamura T, Burian D, Khalili H et al. (2001). "Cloning and characterization of ELL-associated proteins EAP45 and EAP20. a role for yeast EAP-like proteins in regulation of gene expression by glucose.". J. Biol. Chem. 276 (19): 16528–33. doi:10.1074/jbc.M010142200. PMID 11278625.
- Strausberg RL, Feingold EA, Grouse LH et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences.". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- von Schwedler UK, Stuchell M, Müller B et al. (2003). "The protein network of HIV budding.". Cell 114 (6): 701–13. doi:10.1016/S0092-8674(03)00714-1. PMID 14505570.
- Martin-Serrano J, Yarovoy A, Perez-Caballero D et al. (2003). "Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins.". Proc. Natl. Acad. Sci. U.S.A. 100 (21): 12414–9. doi:10.1073/pnas.2133846100. PMC 218772. PMID 14519844.
- Sharma M, Pampinella F, Nemes C et al. (2004). "Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes.". J. Cell Biol. 164 (6): 923–33. doi:10.1083/jcb.200312018. PMC 2172283. PMID 15007060.
- Gerhard DS, Wagner L, Feingold EA et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC).". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Rual JF, Venkatesan K, Hao T et al. (2005). "Towards a proteome-scale map of the human protein-protein interaction network.". Nature 437 (7062): 1173–8. doi:10.1038/nature04209. PMID 16189514.
- Bowers K, Piper SC, Edeling MA et al. (2006). "Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII.". J. Biol. Chem. 281 (8): 5094–105. doi:10.1074/jbc.M508632200. PMID 16371348.
This article incorporates text from the public domain Pfam and InterPro IPR008570