Uterine fibroid
| Uterine fibroids | |
|---|---|
 Uterine Fibroids | |
| Classification and external resources | |
| ICD-10 | D25 |
| ICD-9 | 218.9 |
| OMIM | 150699 |
| DiseasesDB | 4806 |
| MedlinePlus | 000914 |
| eMedicine | radio/777 |
| Patient UK | Uterine fibroid |
| MeSH | D007889 |
A uterine fibroid (also known as uterine leiomyoma,[1] myoma, fibromyoma, fibroleiomyoma) is a leiomyoma (benign tumor from smooth muscle tissue) that originates from the smooth muscle layer (myometrium) of the uterus. Fibroids are often multiple and if the uterus contains too many leiomyomata to count, it is referred to as diffuse uterine leiomyomatosis. The cancerous version of a fibroid is extremely uncommon and termed a leiomyosarcoma.
Fibroids are the most common benign tumors in females and typically found during the middle and later reproductive years. While most fibroids are asymptomatic, they can grow and cause heavy and painful menstruation, painful sexual intercourse, urinary frequency and urgency. Some fibroids may interfere with pregnancy although this appears to be uncommon.[2]
In the United States, symptoms caused by uterine fibroids are a very frequent indication for surgical removal of the uterus.[3]
Signs and symptoms
Fibroids, particularly when small, may be entirely asymptomatic. Symptoms depend on the location of the lesion and its size. Important symptoms include abnormal uterine bleeding, heavy or painful periods, abdominal discomfort or bloating, painful defecation, back ache, urinary frequency or retention, and in some cases, infertility.[4] There may also be pain during intercourse, depending on the location of the fibroid. During pregnancy they may also be the cause of miscarriage, bleeding, premature labor, or interference with the position of the fetus.
While fibroids are common, they are not a typical cause for infertility accounting for about 3% of reasons why a woman may not be able to have a child.[5] The majority of women with uterine fibroids will have normal pregnancy outcomes.[6][7] In cases of intercurrent uterine fibroids in infertility, a fibroid is typically located in a submucosal position and it is thought that this location may interfere with the function of the lining and the ability of the embryo to implant.[5] Also larger fibroids may distort or block the fallopian tubes.
Cause
Genetics
An association with fatty acid synthase has been reported.[8]
Familial leiomyomata
A syndrome (Reed's syndrome) that causes uterine leiomyomata along with cutaneous leiomyomata and renal cell cancer has been reported.[9][10][11] This is associated with a mutation in the gene that produces the enzyme fumarate hydratase, located on the long arm of chromosome 1 (1q42.3-43). Inheritance is autosomal dominant.
Pathophysiology

Leiomyomata grossly appear as round, well circumscribed (but not encapsulated), solid nodules that are white or tan, and show whorled appearance on histological section. The size varies, from microscopic to lesions of considerable size. Typically lesions the size of a grapefruit or bigger are felt by the patient herself through the abdominal wall.

Microscopically, tumor cells resemble normal cells (elongated, spindle-shaped, with a cigar-shaped nucleus) and form bundles with different directions (whorled). These cells are uniform in size and shape, with scarce mitoses. There are three benign variants: bizarre (atypical); cellular; and mitotically active.
The appearance of prominent nucleoli with perinucleolar halos should alert the pathologist to investigate the possibility of the extremely rare hereditary leiomyomatosis and renal cell cancer (Reed) syndrome.[12]
Location and classification

Growth and location are the main factors that determine if a fibroid leads to symptoms and problems.[3] A small lesion can be symptomatic if located within the uterine cavity while a large lesion on the outside of the uterus may go unnoticed. Different locations are classified as follows:
- Intramural fibroids are located within the wall of the uterus and are the most common type; unless large, they may be asymptomatic. Intramural fibroids begin as small nodules in the muscular wall of the uterus. With time, intramural fibroids may expand inwards, causing distortion and elongation of the uterine cavity.
- Subserosal fibroids are located underneath the mucosal (peritoneal) surface of the uterus and can become very large. They can also grow out in a papillary manner to become pedunculated fibroids. These pedunculated growths can actually detach from the uterus to become a parasitic leiomyoma.
- Submucosal fibroids are located in the muscle beneath the endometrium of the uterus and distort the uterine cavity; even small lesions in this location may lead to bleeding and infertility. A pedunculated lesion within the cavity is termed an intracavitary fibroid and can be passed through the cervix.
- Cervical fibroids are located in the wall of the cervix (neck of the uterus). Rarely, fibroids are found in the supporting structures (round ligament, broad ligament, or uterosacral ligament) of the uterus that also contain smooth muscle tissue.
Fibroids may be single or multiple. Most fibroids start in the muscular wall of the uterus. With further growth, some lesions may develop towards the outside of the uterus or towards the internal cavity. Secondary changes that may develop within fibroids are hemorrhage, necrosis, calcification, and cystic changes.
Extrauterine fibroids of uterine origin, metastatic fibroids
Fibroids of uterine origin located in other parts of the body, sometimes also called parasitic myomas have been historically extremely rare, but are now diagnosed with increasing frequency. They may be related or identical to metastasizing leiomyoma.
They are in most cases still hormone dependent but may cause life-threatening complications when they appear in distant organs. Some sources suggest that a substantial share of the cases may be late complications of surgeries such as myomectomy or hysterectomy. Particularly laparoscopic myomectomy using a morcellator has been associated with a substantially increased risk of this complication.[13][14][15][16][17]
There are a number of rare conditions in which fibroids metastasize. They still grow in a benign fashion, but can be dangerous depending on their location.[18]
- In leiomyoma with vascular invasion, an ordinary-appearing fibroid invades into a vessel but there is no risk of recurrence.
- In intravenous leiomyomatosis, leiomyomata grow in veins with uterine fibroids as their source. Involvement of the heart can be fatal.
- In benign metastasizing leiomyoma, leiomyomata grow in more distant sites such as the lungs and lymph nodes. The source is not entirely clear. Pulmonary involvement can be fatal.
- In disseminated intraperitoneal leiomyomatosis, leiomyomata grow diffusely on the peritoneal and omental surfaces, with uterine fibroids as their source. This can simulate a malignant tumor but behaves benignly.
Pathogenesis

Fibroids are monoclonal tumors and approximately 40 to 50% show karyotypically detectable chromosomal abnormalities. When multiple fibroids are present they frequently have unrelated genetic defects. Specific mutations of the MED12 protein have been noted in 70 percent of fibroids.[19]
The exact cause of fibroids is not clearly understood, but the current working hypothesis is that genetic predispositions, prenatal hormone exposure and the effects of hormones, growth factors and xenoestrogens cause fibroid growth. Known risk factors are African descent, obesity, polycystic ovary syndrome, diabetes, hypertension, and never having given birth.[20]
Fibroid growth is strongly dependent on estrogen and progesterone. Although both estrogen and progesterone are usually regarded as growth-promoting they will also cause growth restriction in some circumstances. Paradoxically, fibroids rarely grow during pregnancy despite very high steroid hormone levels and pregnancy appears to exert a certain protective effect.[2] This protective effect might be partially mediated by an interaction between estrogen and the oxytocin receptor.[21]
It is believed that estrogen and progesterone have a mitogenic effect on leiomyoma cells and also act by influencing (directly and indirectly) a large number of growth factors, cytokines and apoptotic factors as well as other hormones. Furthermore, the actions of estrogen and progesterone are modulated by the cross-talk between estrogen, progesterone and prolactin signalling which controls the expression of the respective nuclear receptors. It is believed that estrogen promotes growth by up-regulating IGF-1, EGFR, TGF-beta1, TGF-beta3 and PDGF, and promotes aberrant survival of leiomyoma cells by down-regulating p53, increasing expression of the anti-apoptotic factor PCP4 and antagonizing PPAR-gamma signalling. Progesterone is thought to promote the growth of leiomyoma through up-regulating EGF, TGF-beta1 and TGF-beta3, and promotes survival through up-regulating Bcl-2 expression and down-regulating TNF-alpha. Progesterone is believed to counteract growth by downregulating IGF-1.[22][23][24] Expression of transforming growth interacting factor (TGIF) is increased in leiomyoma compared with myometrium.[25] TGIF is a potential repressor of TGF-β pathways in myometrial cells.[25]
Whereas in premenopausal fibroids the ER-beta, ER-alpha and progesterone receptors are found overexpressed, in the rare postmenopausal fibroids only ER-beta was found significantly overexpressed.[26] Most studies found that polymorphisms in ER and PR gene encodings are not correlated with incidence of fibroids in Caucasian populations[27][28] however a special ER-alpha genotype was found correlated with incidence and size of fibroids. The higher prevalence of this genotype in black women may also explain the high incidence of fibroids in this group.[29]
Uterine leiomyoma was more sensitive than normal myometrium to PPAR-gamma receptor activation resulting in reduced survival and apoptosis of leiomyoma cells. The mechanism is thought to involve negative cross-talk between ER and PPAR signaling pathways. Several PPAR-gamma ligands were considered as potential treatment.[30] PPAR-gamma agonists may also counteract leiomyoma growth by several other mechanisms of action such as TGF-beta3 expression inhibition.[31]
Aromatase and 17beta-hydroxysteroid dehydrogenase are aberrantly expressed in fibroids, indicating that fibroids can convert circulating androstenedione into estradiol.[32] Similar mechanism of action has been elucidated in endometriosis and other endometrial diseases.[33] Aromatase inhibotors are currently considered for treatment, at certain doses they would completely inhibit estrogen production in the fibroid while not largely affecting ovarian production of estrogen (and thus systemic levels of it). Aromatase overexpression is particularly pronounced in Afro-American women.[34]
Genetic and hereditary causes are being considered and several epidemiologic findings indicate considerable genetic influence especially for early onset cases. First degree relatives have a 2.5-fold risk, and nearly 6-fold risk when considering early onset cases. Monozygotic twins have double concordance rate for hysterectomy compared to dizygotic twins.[35]
Expansion of uterine fibroids is by a slow rate of cell proliferation combined with the production of copious amounts of extracellular matrix.[36] Recent studies suggest that this production may represent an abnormal response to ischemic and mechanical tissue stress.[37] Several factors indicate significant involvement of extracellular signaling pathways such as ERK1 and ERK2, which in fibroids are prominently influenced by hormones.[38] Paradoxically and unlike most other conditions involving significant fibrosis the CYR61 gene has been found downregulated in fibroids.[39]
Cyr61 is also known for its role as tumor suppressing factor and in angiogenesis. Hence fibroids are one of the very few tumors with reduced vascular density.[39]
A small population of the cells in an uterine fibroid have properties of stem cells or progenitor cells, and contribute significantly to ovarian steroid-dependent growth of fibroids. These stem-progenitor cells are deficient in estrogen receptor α and progesterone receptor and instead rely on substantially higher levels of these receptors in surrounding differentiated cells to mediate estrogen and progesterone actions via paracrine signalling.[36]
Diagnosis
While a bimanual examination typically can identify the presence of larger fibroids, gynecologic ultrasonography (ultrasound) has evolved as the standard tool to evaluate the uterus for fibroids. Sonography will depict the fibroids as focal masses with a heterogeneous texture, which usually cause shadowing of the ultrasound beam. The location can be determined and dimensions of the lesion measured. Also magnetic resonance imaging (MRI) can be used to define the depiction of the size and location of the fibroids within the uterus.
Imaging modalities cannot clearly distinguish between the benign uterine leiomyoma and the malignant uterine leiomyosarcoma, however, the latter is quite rare. Fast growth or unexpected growth, such as enlargement of a lesion after menopause, raise the level of suspicion that the lesion might be a sarcoma. Also, with advanced malignant lesions there may be evidence of local invasion. A more recent study has suggested that diagnostic capabilities using MRI have improved the ability to detect sarcomatous lesions.[40] Biopsy is rarely performed and if performed, is rarely diagnostic. Should there be an uncertain diagnosis after ultrasounds and MRI imaging, surgery is generally indicated.
Other imaging techniques that may be helpful specifically in the evaluation of lesions that affect the uterine cavity are hysterosalpingography or sonohysterography.
-

A very large (9 cm) fibroid of the uterus which is causing pelvic congestion syndrome as seen on CT
-

A very large (9 cm) fibroid of the uterus which is causing pelvic congestion syndrome as seen on ultrasound
-

A relatively large submucosal leiomyoma; it fills out the major part of the endometrial cavity
-
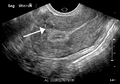
A small uterine fibroid seen within the wall of the myometrium on a cross sectional ultrasound view
-
Two calcified fibroids (in the uterus)
-

thumb|A subserosal uterine fibroid with a diameter of 5 centimeters.
Coexisting disorders
Fibroids that lead to heavy vaginal bleeding lead to anemia and iron deficiency. Due to pressure effects gastrointestinal problems such as constipation and bloatedness are possible. Compression of the ureter may lead to hydronephrosis. Fibroids may also present alongside endometriosis, which itself may cause infertility. Adenomyosis may be mistaken for or coexist with fibroids.
In very rare cases, malignant (cancerous) growths, leiomyosarcoma, of the myometrium can develop.[41] In extremely rare cases uterine fibroids may present as part or early symptom of the hereditary leiomyomatosis and renal cell cancer syndrome.
Treatment
Most fibroids do not require treatment unless they are causing symptoms. After menopause fibroids shrink and it is unusual for them to cause problems. In those who have symptoms uterine artery embolization and surgical options have similar outcomes with respect to satisfaction.[42]
Symptomatic uterine fibroids can be treated by:
- medication to control symptoms
- medication aimed at shrinking tumours
- ultrasound fibroid destruction
- myomectomy or radio frequency ablation
- hysterectomy
- uterine artery embolization
Medication
A number of medications may be used to control symptoms. NSAIDs can be used to reduce painful menses. Oral contraceptive pills are prescribed to reduce uterine bleeding and cramps.[5] Anemia may have to be treated with iron supplementation. Vitamin D3 supplementation can be tried.[43][44][45]
Levonorgestrel intrauterine devices are highly effective in limiting menstrual blood flow and improving other symptoms. Side effects are typically very moderate because the levonorgestrel (a progestin) is released in low concentration locally. There is now substantial evidence that Levongestrel-IUDs provide good symptomatic relief for women with fibroids.[46] While most Levongestrel-IUD studies concentrated on treatment of women without fibroids a few reported very good results specifically for women with fibroids including a substantial regression of fibroids.[47][48][49]
Dostinex in a moderate and well tolerated dosis has been shown in 2 studies to shrink fibroids effectively. Mechanism of action is unclear.[48][50]
Ulipristal acetate is a synthetic selective progesterone receptor modulator which has been tested in several randomized trials with good results for the treatment of fibroids.[51][52] Similar to other selective progesterone receptor modulators and antagonists benign histologic endometrial changes were reported and long term safety outside of clinical studies has not been established yet.[51][53][54]
Danazol is an effective treatment to shrink fibroids and control symptoms. Its use is limited by unpleasant side effects. Mechanism of action is thought to be antiestrogenic effects. Recent experience indicates that safety and side effect profile can be improved by more cautious dosing.[48]
Gonadotropin-releasing hormone analogs cause temporary regression of fibroids by decreasing estrogen levels. Because of the limitations and side effects of this medication it is rarely recommended other than for preoperative use to shrink the size of the fibroids and uterus before surgery. It is typically used for a maximum of 6 months or less because after longer use they could cause osteoporosis and other typically postmenopausal complications. The main side effects are transient postmenopausal symptoms. In many cases the fibroids will regrow after cessation of treatment, however significant benefits may persist for much longer in some cases. Several variations are possible, such as GnRH agonists with add-back regimens intended to decrease the adverse effects of estrogen deficiency. Several add-back regimes are possible, tibolone, raloxifene, progestogens alone, estrogen alone, and combined estrogens and progestogens.[48]
Progesterone antagonists such as Mifepristone have been tested, there is evidence that it relieves some symptoms and improves quality of life but because of adverse histological changes that have been observed in several trials it can not be currently recommended outside of research setting.[55][56][57] Fibroid growth has recurred after antiprogestin treatment was stopped.[36] Selective progesterone receptor modulators, such as Progenta, have been under investigation.
The selective progesterone receptor modulator Asoprisnil is currently tested with very promising results as a possible use as a treatment for fibroids - the hope is that it will provide the advantages of progesterone antangonitst without their adverse effects.[48]
The long term safety of progesterone antagonists as well as selective progesterone receptor modulators has yet to be established.[58]
Aromatase inhibitors have been used experimentally to reduce fibroids. The effect is believed to be due partially by lowering systemic estrogen levels and partially by inhibiting locally overexpressed aromatase in fibroids.[48] However, fibroid growth has recurred after treatment was stopped.[36] Experience from experimental aromatase inhibitor treatment of endometriosis indicates that aromatase inhibitors might be particularly useful in combination with a progestogenic ovulation inhibitor.
Uterine artery embolization
Uterine artery embolization (UAE): is a noninvasive, endovascular procedure effectively treating symptomatic fibroids. Using interventional radiology techniques, the interventional radiologist occludes both uterine arteries, thus reducing blood supply to the fibroid.[59] This intervention is not usually recommended when fertility should be preserved although subsequent pregnancies are usually possible. A small catheter (1 mm in diameter) is inserted into the femoral artery at the level of the groin under local anesthesia. Under imaging guidance, the interventional radiologist will enter selectively into both uterine arteries and inject small (500 µm) particles that will block the blood supply to the fibroids. A patient will usually recover from the procedure within a few days. The UAE procedure should result in limited blood supply to the fibroids which should prevent them from further growth, heavy bleeding and possibly shrink them.
In 1994, Dr. Bruce McLucas pioneered the first successful Uterine Artery Embolization (UAE) procedure in the United States. Since then, he has successfully performed UAE on thousands of patients worldwide. He is one of the few gynecologists in the world with the expertise to perform UAE. Dr. McLucas also trains physicians throughout the world to successfully perform UAE.
Uterine artery ligation
Uterine artery ligation, sometimes also laparoscopic occlusion of uterine arteries are minimally invasive methods to limit blood supply of the uterus by a small surgery that can be performed transvaginally or laparoscopically. The principal mechanism of action may be similar like in UAE but is easier to perform and fewer side effects are expected.[60][61][62] UAE currently appears much more effective than this method in direct comparison.[63]
Radio frequency ablation
Radiofrequency ablation is a minimally invasive treatments for fibroids.[64] In this technique the fibroid is shrunk by inserting a needle-like device into the fibroid through the abdomen and heating it with radio-frequency (RF) electrical energy to cause necrosis of cells. The treatment is a potential option for women who have fibroids, have completed child-bearing and want to avoid a hysterectomy.
Myomectomy



Myomectomy is a surgery to remove one or more fibroids. It is usually recommended when more conservative treatment options fail for women who want fertility preserving surgery or who want to retain the uterus.[65]
There are three types of myomectomy:
- In a hysteroscopic myomectomy (also called transcervical resection), the fibroid can be removed by either the use of a resectoscope, an endoscopic instrument inserted through the vagina and cervix that can use high-frequency electrical energy to cut tissue, or a similar device.
- A laparoscopic myomectomy is done through a small incision near the navel. The physician uses a laparoscope and surgical instruments to remove the fibroids. Studies have suggested that laparoscopic myomectomy leads to lower morbidity rates and faster recovery than does laparotomic myomectomy.[66]
- A laparotomic myomectomy (also known as an open or abdominal myomectomy) is the most invasive surgical procedure to remove fibroids. The physician makes an incision in the abdominal wall and removes the fibroids from the uterus.
Laparoscopic myomectomy has less pain and shower time in hospital than open surgery.[67]
Hysterectomy
Hysterectomy was the classical method of treating fibroids. Although it is now recommended only as last option, fibroids are still the leading cause of hysterectomies in the US.
Endometrial ablation
Endometrial ablation can be used if the fibroids are only within the uterus and not intramural and relatively small. High failure and recurrence rates are expected in the presence of larger or intramural fibroids.
Magnetic resonance guided focused ultrasound
Magnetic resonance guided focused ultrasound, is a non-invasive intervention (requiring no incision) that uses high intensity focused ultrasound waves to destroy tissue in combination with magnetic resonance imaging (MRI), which guides and monitors the treatment. During the procedure, delivery of focused ultrasound energy is guided and controlled using MR thermal imaging.[68] Patients who have symptomatic fibroids, who desire a non-invasive treatment option and who do not have contraindictions for MRI are candidates for MRgFUS. About 60% of patients qualify. It is an outpatient procedure and takes one to three hours depending on the size of the fibroids. It is safe and about 75% effective.[69] Symptomatic improvement is sustained for two plus years.[70] Need for additional treatment varies from 16-20% and is largely dependent on the amount of fibroid that can be safely ablated; the higher the ablated volume, the lower the re-treatment rate.[71] In comparison to available treatment options, the cost effectiveness of MRgFUS in the U.S. and U.K. has been found to be reasonable and comparable to alternative treatments (hysterectomy, pharmacotherapy, uterine artery embolization).[72][73] There are currently no randomized trial between MRgFUS and UAE. A multi-center trial is underway to investigate the efficacy of MRgFUS vs. UAE.
Prognosis
About 1 out of 1000 lesions[5] are or become malignant, typically as a leiomyosarcoma on histology. A sign that a lesion may be malignant is growth after menopause.[5] There is no consensus among pathologists regarding the transformation of leiomyoma into a sarcoma.
Metastasis
There are a number of rare conditions in which fibroids metastasize. They still grow in a benign fashion, but can be dangerous depending on their location.[18]
Epidemiology
Globally approximately 235 million people are affected with uterine fibroids as of 2010 (6.6% of females).[74] About 20–40% of women will be diagnosed with leiomyoma at some point in their life but only a fraction of those will cause problems or require treatment.[3]
Leiomyomata are more common in obese women.[75] Fibroids are dependent on estrogen and progesterone to grow and therefore relevant only during the reproductive years, they are expected to shrink after menopause.
United States
Eighty percent of African American women will develop benign uterine fibroid tumors by their late 40s, according to the National Institute of Environmental Health Sciences.[76] African American women are two to three times more likely to get fibroids than Caucasian women.[75][77][78] In African-American women fibroids seem to occur at a younger age, grow more quickly, and are more likely to cause symptoms.[79] This leads to higher rates of surgery for African Americans, both myomectomy and hysterectomy.[80] Increased risk of fibroids in African- Americans causes them to fare worse in in-vitro fertility treatments and raises their risk of premature births and delivery by Cesarean section.[80]
It is unclear why fibroids are more common in African American women. Some studies suggest that black women who are obese and who have high blood pressure are more likely to have fibroids.[80] Black women consume fewer servings of dairy than white women and have lower intake of calcium, magnesium and phosphorus, while some data suggest that increased dairy intake in African American women is associated with lower risk of fibroids.[81] A relation between the use of hair relaxers and risk of developing fibroids has been found, high prevalence of the use of hair relaxer among African American women may explain some of the risk.[82]
Society and culture
United States law
The 2005 S.1289 bill was read twice and referred to the committee on Health, Labor and Pensions but never passed for a Senate or House vote. The proposed Uterine Fibroid Research and Education Act of 2005 mentioned that $5 billion is spent annually on hysterectomy surgeries each year, which affect 22% of African Americans and 7% of Caucasian women. The bill also called for more funding for research and educational purposes. It also states that of the $28 billion issued to NIH,[83] $5 million was allocated for uterine fibroids in 2004.
Other animals
Uterine fibroids are rare in other mammals, although they have been observed in certain dogs and Baltic grey seals.[84]
References
- ↑ "uterine leiomyoma" at Dorland's Medical Dictionary
- ↑ 2.0 2.1 Neiger R, Sonek JD, Croom CS, Ventolini G (2006). "Pregnancy-related changes in the size of uterine leiomyomas". The Journal of reproductive medicine 51 (9): 671–674. PMID 17039693.
- ↑ 3.0 3.1 3.2 Wallach EE, Vlahos NF (August 2004). "Uterine myomas: an overview of development, clinical features, and management". Obstet Gynecol 104 (2): 393–406. doi:10.1097/01.AOG.0000136079.62513.39. PMID 15292018.
- ↑ "Benign Uterine Fibroid Tumors (What to Know)". Women's Health. about.com.
- ↑ 5.0 5.1 5.2 5.3 5.4 American Society of Reproductive Medicine Patient Booklet: Uterine Fibroids, 2003
- ↑ Segars JH, Parrott EC, Nagel JD, Guo XC, Gao X, Birnbaum LS et al. (2014). "Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations". Human Reproduction Update 20 (3): 309–333. doi:10.1093/humupd/dmt058. PMC 3999378. PMID 24401287.
- ↑ Segars JH, Parrott EC, Nagel JD, Guo XC, Gao X, Birnbaum LS et al. (2014). "Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations". Hum. Reprod. Update 20 (3): 309–33. doi:10.1093/humupd/dmt058. PMC 3999378. PMID 24401287.
- ↑ Eggert SL, Huyck KL, Somasundaram P, Kavalla R, Stewart EA, Lu AT et al. (October 2012). "Genome-wide linkage and association analyses implicate FASN in predisposition to Uterine Leiomyomata". Am. J. Hum. Genet. 91 (4): 621–8. doi:10.1016/j.ajhg.2012.08.009. PMC 3484658. PMID 23040493.
- ↑ Tolvanen J, Uimari O, Ryynänen M, Aaltonen LA, Vahteristo P (2012). "Strong family history of uterine leiomyomatosis warrants fumarate hydratase mutation screening". Human Reproduction 27 (6): 1865–9. doi:10.1093/humrep/des105. PMID 22473397.
- ↑ Toro JR et al. (2003). "Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America". Am J Hum Genet 73 (1): 95–106. doi:10.1086/376435. PMC 1180594. PMID 12772087.
- ↑ http://rarediseases.info.nih.gov/GARD/Condition/10160/Reed_syndrome.aspx[]
- ↑ Garg K, Tickoo SK, Soslow RA, Reuter VE (2011). "Morphologic Features of Uterine Leiomyomas Associated with Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome". The American Journal of Surgical Pathology 35 (8): 1235–1237. doi:10.1097/PAS.0b013e318223ca01. PMID 21753700.
- ↑ Cucinella G, Granese R, Calagna G, Somigliana E, Perino A (2011). "Parasitic myomas after laparoscopic surgery: An emerging complication in the use of morcellator? Description of four cases". Fertility and Sterility 96 (2): e90–e96. doi:10.1016/j.fertnstert.2011.05.095. PMID 21719004.
- ↑ Nezhat C, Kho K (2010). "Iatrogenic Myomas: New Class of Myomas?". Journal of Minimally Invasive Gynecology 17 (5): 544–550. doi:10.1016/j.jmig.2010.04.004. PMID 20580324.
- ↑ Kumar_2008Kumar S, Sharma JB, Verma D, Gupta P, Roy KK, Malhotra N (2008). "Disseminated peritoneal leiomyomatosis: An unusual complication of laparoscopic myomectomy". Archives of Gynecology and Obstetrics 278 (1): 93–95. doi:10.1007/s00404-007-0536-9. PMID 18193441.
- ↑ Galvin SD, Wademan B, Chu J, Bunton RW (2010). "Benign Metastasizing Leiomyoma: A Rare Metastatic Lesion in the Right Ventricle". The Annals of Thoracic Surgery 89 (1): 279–281. doi:10.1016/j.athoracsur.2009.06.050. PMID 20103256.
- ↑ Mikami I, Yamamoto M, Nishimura H, Koizumi K, Gomibuchi M, Tanaka S (1998). "Multiple lungs tumors found 17 years after hysterectomy--a case of benign metastasizing leiomyoma". The Japanese journal of thoracic and cardiovascular surgery : official publication of the Japanese Association for Thoracic Surgery = Nihon Kyobu Geka Gakkai zasshi 46 (7): 634–638. doi:10.1007/BF03217793. PMID 9750447.
- ↑ 18.0 18.1 Fletcher's Diagnostic Histopathology of Tumors (3rd ed.). pp. 692–4.
- ↑ Mäkinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ et al. (2011). "MED12, the Mediator Complex Subunit 12 Gene, is Mutated at High Frequency in Uterine Leiomyomas". Science 334 (6053): 252–255. Bibcode:2011Sci...334..252M. doi:10.1126/science.1208930. PMID 21868628.
- ↑ Okolo S (2008). "Incidence, aetiology and epidemiology of uterine fibroids". Best practice & research. Clinical obstetrics & gynaecology 22 (4): 571–588. doi:10.1016/j.bpobgyn.2008.04.002. PMID 18534913.
- ↑ Cesen-Cummings K, Houston KD, Copland JA, Moorman VJ, Walker CL, Davis BJ (2003). "Uterine leiomyomas express myometrial contractile-associated proteins involved in pregnancy-related hormone signaling". Journal of the Society for Gynecologic Investigation 10 (1): 11–20. doi:10.1016/S1071-5576(02)00191-0. PMID 12517588.
- ↑ Rein MS (2000). "Advances in uterine leiomyoma research: the progesterone hypothesis". Environmental health perspectives. 108 Suppl 5: 791–3. doi:10.2307/3454308. JSTOR 3454308. PMID 11035984.
- ↑ Maruo T, Ohara N, Wang J, Matsuo H (2004). "Sex steroidal regulation of uterine leiomyoma growth and apoptosis". Human reproduction update 10 (3): 207–220. doi:10.1093/humupd/dmh019. PMID 15140868.
- ↑ Wei T, Geiser AG, Qian HR, Su C, Helvering LM, Kulkarini NH et al. (2007). "DNA microarray data integration by ortholog gene analysis reveals potential molecular mechanisms of estrogen-dependent growth of human uterine fibroids". BMC Women's Health 7: 5. doi:10.1186/1472-6874-7-5. PMC 1852551. PMID 17407572.
- ↑ 25.0 25.1 Yen-Ping Ho J, Man WC, Wen Y, Polan ML, Shih-Chu Ho E, Chen B (June 2009). "Transforming growth interacting factor expression in leiomyoma compared with myometrium". Fertil. Steril. 94 (3): 1078–83. doi:10.1016/j.fertnstert.2009.05.001. PMC 2888713. PMID 19524896.
- ↑ Strissel PL, Swiatek J, Oppelt P, Renner SP, Beckmann MW, Strick R (2007). "Transcriptional analysis of steroid hormone receptors in smooth muscle uterine leiomyoma tumors of postmenopausal patients". The Journal of Steroid Biochemistry and Molecular Biology 107 (1–2): 42–47. doi:10.1016/j.jsbmb.2007.02.005. PMID 17646097.
- ↑ Massart F, Becherini L, Marini F, Noci I, Piciocchi L, Del Monte F et al. (2003). "Analysis of estrogen receptor (ERalpha and ERbeta) and progesterone receptor (PR) polymorphisms in uterine leiomyomas". Medical science monitor : international medical journal of experimental and clinical research 9 (1): BR25–BR30. PMID 12552233.
- ↑ Fischer C, Juhasz-Boess I, Lattrich C, Ortmann O, Treeck O (2010). "Estrogen receptor β gene polymorphisms and susceptibility to uterine fibroids". Gynecological Endocrinology 26 (1): 4–9. doi:10.3109/09513590903159573. PMID 19639498.
- ↑ Al-Hendy A, Salama SA (2006). "Ethnic distribution of estrogen receptor-α polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans". Fertility and Sterility 86 (3): 686–93. doi:10.1016/j.fertnstert.2006.01.052. PMID 16860797.
- ↑ Nam DH, Ramachandran S, Song DK, Kwon KY, Jeon DS, Shin SJ et al. (2007). "Growth inhibition and apoptosis induced in human leiomyoma cells by treatment with the PPAR gamma ligand ciglitizone". Molecular Human Reproduction 13 (11): 829–836. doi:10.1093/molehr/gam071. PMID 17893092.
- ↑ Zhang CH, Wen ZQ, Li JF, Li CZ, Shi M, Yang GW et al. (2008). "Inhibition of proliferation and transforming growth factor beta3 protein expression by peroxisome proliferators-activated receptor gamma ligands in human uterine leiomyoma cells". Chinese medical journal 121 (2): 166–171. PMID 18272045.
- ↑ Shozu M, Murakami K, Inoue M (2004). "Aromatase and Leiomyoma of the Uterus". Seminars in Reproductive Medicine 22 (1): 51–60. doi:10.1055/s-2004-823027. PMID 15083381.
- ↑ Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Zhou J et al. (2001). "Role of aromatase in endometrial disease". The Journal of Steroid Biochemistry and Molecular Biology 79 (1–5): 19–25. doi:10.1016/S0960-0760(01)00134-0. PMID 11850203.
- ↑ Ishikawa H, Reierstad S, Demura M, Rademaker AW, Kasai T, Inoue M et al. (2009). "High Aromatase Expression in Uterine Leiomyoma Tissues of African-American Women". Journal of Clinical Endocrinology & Metabolism 94 (5): 1752–1756. doi:10.1210/jc.2008-2327. PMC 2684481. PMID 19240151.
- ↑ Hodge JC, Morton CC (2007). "Genetic heterogeneity among uterine leiomyomata: insights into malignant progression". Human Molecular Genetics. 16 Spec No 1: R7–13. doi:10.1093/hmg/ddm043. PMID 17613550.
- ↑ 36.0 36.1 36.2 36.3 Moravek MB, Yin P, Ono M, Coon V JS, Dyson MT, Navarro A et al. (2014). "Ovarian steroids, stem cells and uterine leiomyoma: therapeutic implications". Human Reproduction Update 21 (1): 1–12. doi:10.1093/humupd/dmu048. PMID 25205766.
- ↑ Payson M, Malik M, Siti-Nur Morris S, Segars JH, Chason R, Catherino WH (2009). "Activating transcription factor 3 gene expression suggests that tissue stress plays a role in leiomyoma development". Fertil Steril. 92 (2): 748–55. doi:10.1016/j.fertnstert.2008.06.030. PMC 4130340. PMID 18692824.
- ↑ Nierth-Simpson EN, Martin MM, Chiang TC, Melnik LI, Rhodes LV, Muir SE et al. (2009). "Human uterine smooth muscle and leiomyoma cells differ in their rapid 17beta-estradiol signaling: implications for proliferation". Endocrinology 150 (5): 2436–2445. doi:10.1210/en.2008-0224. PMC 2671893. PMID 19179429.
- ↑ 39.0 39.1 Weston G, Trajstman AC, Gargett CE, Manuelpillai U, Vollenhoven BJ, Rogers PA (2003). "Fibroids display an anti-angiogenic gene expression profile when compared with adjacent myometrium". Molecular human reproduction 9 (9): 541–549. doi:10.1093/molehr/gag066. PMID 12900513.
- ↑ Goto A, Takeuchi S, Sugimura K, Maruo T (2002). "Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus". Int. J. Gynecol. Cancer 12 (4): 354–61. doi:10.1046/j.1525-1438.2002.01086.x. PMID 12144683.
- ↑ "Fibroids". NHS Choices. U.K. National Health Service.
- ↑ Gupta JK, Sinha A, Lumsden MA, Hickey M (26 December 2014). "Uterine artery embolization for symptomatic uterine fibroids.". The Cochrane database of systematic reviews 12: CD005073. doi:10.1002/14651858.CD005073.pub4. PMID 25541260.
- ↑ Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A (2013). "Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study". International Journal of Women's Health 5: 93–100. doi:10.2147/IJWH.S38800. PMC 3589082. PMID 23467803.
- ↑ Paffoni A, Somigliana E, Vigano' P, Benaglia L, Cardellicchio L, Pagliardini L et al. (2013). "Vitamin D status in women with uterine leiomyomas". The Journal of Clinical Endocrinology & Metabolism 98 (8): E1374–8. doi:10.1210/jc.2013-1777. PMID 23824422.
- ↑ Halder SK, Sharan C, Al-Hendy A (2012). "1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model". Biology of Reproduction 86 (4): 116. doi:10.1095/biolreprod.111.098145. PMC 3338660. PMID 22302692.
- ↑ Zapata LB, Whiteman MK, Tepper NK, Jamieson DJ, Marchbanks PA, Curtis KM (2010). "Intrauterine device use among women with uterine fibroids: a systematic review☆". Contraception 82 (1): 41–55. doi:10.1016/j.contraception.2010.02.011. PMID 20682142.
- ↑ Jindabanjerd K, Taneepanichskul S (2006). "The use of levonorgestrel - IUD in the treatment of uterine myoma in Thai women". Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 89 Suppl 4: S147–51. PMID 17726816.
- ↑ 48.0 48.1 48.2 48.3 48.4 48.5 Sankaran S, Manyonda IT (2008). "Medical management of fibroids" (PDF). Best Pract Res Clin Obstet Gynaecol 22 (4): 655–76. doi:10.1016/j.bpobgyn.2008.03.001. PMID 18468953.
- ↑ Kailasam C, Cahill D (2008). "Review of the safety, efficacy and patient acceptability of the levonorgestrel-releasing intrauterine system". Patient preference and adherence 2: 293–302. doi:10.2147/ppa.s3464. PMC 2770406. PMID 19920976.
- ↑ Sayyah-Melli M, Tehrani-Gadim S, Dastranj-Tabrizi A, Gatrehsamani F, Morteza G, Ouladesahebmadarek E et al. (2009). "Comparison of the effect of gonadotropin-releasing hormone agonist and dopamine receptor agonist on uterine myoma growth. Histologic, sonographic, and intra-operative changes". Saudi medical journal 30 (8): 1024–33. PMID 19668882.
- ↑ 51.0 51.1 Nieman LK, Blocker W, Nansel T, Mahoney S, Reynolds J, Blithe D et al. (2011). "Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: A randomized, double-blind, placebo-controlled, phase IIb study". Fertility and Sterility 95 (2): 767–772.e1–772. doi:10.1016/j.fertnstert.2010.09.059. PMC 4180231. PMID 21055739.
- ↑ Donnez J, Vázquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC et al. (2014). "Long-term treatment of uterine fibroids with ulipristal acetate ☆". Fertility and Sterility 101 (6): 1565–73.e1–18. doi:10.1016/j.fertnstert.2014.02.008. PMID 24630081.
- ↑ Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T et al. (2012). "Ulipristal acetate versus placebo for fibroid treatment before surgery". N. Engl. J. Med. 366 (5): 409–20. doi:10.1056/NEJMoa1103182. PMID 22296075.
- ↑ Levens ED, Potlog-Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL et al. (2008). "CDB-2914 for Uterine Leiomyomata Treatment". Obstetrics & Gynecology 111 (5): 1129–1136. doi:10.1097/AOG.0b013e3181705d0e. PMC 2742990. PMID 18448745.
- ↑ Tristan M, Orozco LJ, Steed A, Ramírez-Morera A, Stone P (2012). Orozco LJ, ed. "Mifepristone for uterine fibroids". Cochrane database of systematic reviews (Online) 8: CD007687. doi:10.1002/14651858.CD007687.pub2. PMID 22895965.
- ↑ Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell-Danielsson K (August 2009). "Mifepristone for Treatment of Uterine Leiomyoma. A Prospective Randomized Placebo Controlled Trial". Human Reproduction 24 (8): 1870–9. doi:10.1093/humrep/dep100. PMID 19389793.
- ↑ Malartic C, Morel O, Akerman G, Tulpin L, Desfeux P, Barranger E (2008). "La mifépristone dans la prise en charge des fibromes utérins". Gynécologie Obstétrique & Fertilité 36 (6): 668–74. doi:10.1016/j.gyobfe.2008.01.017. PMID 18539512.
- ↑ Wilkens J, Williams AR, Chwalisz K, Han C, Cameron IT, Critchley HO (2009). "Effect of asoprisnil on uterine proliferation markers and endometrial expression of the tumour suppressor gene, PTEN". Human reproduction (Oxford, England) 24 (5): 1036–44. doi:10.1093/humrep/den494. PMID 19176543.
- ↑ "The Embolisation Process". FEmISA: Fibroid Embolisation: Information, Support, Advice.
- ↑ Liu WM, Ng HT, Wu YC, Yen YK, Yuan CC (2001). "Laparoscopic bipolar coagulation of uterine vessels: a new method for treating symptomatic fibroids". Fertility and Sterility 75 (2): 417–22. doi:10.1016/S0015-0282(00)01724-6. PMID 11172850.
- ↑ Kim HS, Kim JW, Kim MK, Chung HH, Lee TS, Jeon YT et al. (2009). "A randomized prospective trial of the postoperative quality of life between laparoscopic uterine artery ligation and laparoscopy-assisted vaginal hysterectomy for the treatment of symptomatic uterine fibroids: clinical trial design". Trials 10: 8. doi:10.1186/1745-6215-10-8. PMC 2645389. PMID 19178748.
- ↑ Akinola OI, Fabamwo AO, Ottun AT, Akinniyi OA (2005). "Uterine artery ligation for management of uterine fibroids". International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 91 (2): 137–40. doi:10.1016/j.ijgo.2005.07.012. PMID 16168993.
- ↑ Hald K, Noreng HJ, Istre O, Kløw NE (2009). "Uterine Artery Embolization versus Laparoscopic Occlusion of Uterine Arteries for Leiomyomas: Long-term Results of a Randomized Comparative Trial". Journal of Vascular and Interventional Radiology 20 (10): 1303–10; quiz 1311. doi:10.1016/j.jvir.2009.07.022. PMID 19713130.
- ↑ Beck, Melinda (2010-01-20). "A New Treatment to Help Women Avoid Hysterectomy". The Wall Street Journal.
- ↑ Metwally M, Cheong YC, Horne AW (2012). Metwally M, ed. "Surgical treatment of fibroids for subfertility". Cochrane database of systematic reviews (Online) 11: CD003857. doi:10.1002/14651858.CD003857.pub3. PMID 23152222.
- ↑ Agdi M, Tulandi T (August 2008). "Endoscopic management of uterine fibroids". Best Pract Res Clin Obstet Gynaecol 22 (4): 707–16. doi:10.1016/j.bpobgyn.2008.01.011. PMID 18325839.
- ↑ Bhave Chittawar P, Franik S, Pouwer AW, Farquhar C (Oct 21, 2014). "Minimally invasive surgical techniques versus open myomectomy for uterine fibroids.". The Cochrane database of systematic reviews 10: CD004638. doi:10.1002/14651858.CD004638.pub3. PMID 25331441.
- ↑ "FDA Approves New Device to Treat Uterine Fibroids" (Press release). FDA. 2004-10-22. Retrieved 2008-05-26.
- ↑ Shen SH, Fennessy F, McDannold N, Jolesz F, Tempany C (April 2009). "Image-guided thermal therapy of uterine fibroids". Seminars in ultrasound, CT, and MR 30 (2): 91–104. doi:10.1053/j.sult.2008.12.002. PMC 2768544. PMID 19358440.
- ↑ Stewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gostout B et al. (January 2006). "Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids". Fertil. Steril. 85 (1): 22–9. doi:10.1016/j.fertnstert.2005.04.072. PMID 16412721.
- ↑ Kurashvili J, Stepanov A, Kulabuchova E, Batarshina O (2014). "MRgFUS for Uterine Myomas: Safety, Effectiveness and Pathogenesis". Journal of Therapeutic Ultrasound 2 (Suppl 1): A1. doi:10.1186/2050-5736-2-S1-A1.
- ↑ O'Sullivan AK, Thompson D, Chu P, Lee DW, Stewart EA, Weinstein MC (January 2009). "Cost-effectiveness of magnetic resonance guided focused ultrasound for the treatment of uterine fibroids". Int J Technol Assess Health Care 25 (1): 14–25. doi:10.1017/S0266462309090035. PMC 2811401. PMID 19126247.
- ↑ Zowall H, Cairns JA, Brewer C, Lamping DL, Gedroyc WM, Regan L (April 2008). "Cost-effectiveness of magnetic resonance-guided focused ultrasound surgery for treatment of uterine fibroids". BJOG 115 (5): 653–62. doi:10.1111/j.1471-0528.2007.01657.x. PMC 2344162. PMID 18333948.
- ↑ Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M et al. (Dec 15, 2012). "Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet 380 (9859): 2163–96. doi:10.1016/S0140-6736(12)61729-2. PMID 23245607.
- ↑ 75.0 75.1 Uterine Fibroids at Merck Manual of Diagnosis and Therapy Professional Edition
- ↑ "Helping Black Women Recognize, Treat Fibroids". NPR. Retrieved 30 March 2011.
- ↑ "African American Women and Fibroids". Philadelphia Black Women's Health Project. Retrieved 30 March 2011.
- ↑ Wise LA, Palmer JR, Stewart EA, Rosenberg L (March 2005). "Age-specific incidence rates for self-reported uterine leiomyomata in the Black Women's Health Study". Obstet Gynecol 105 (3): 563–8. doi:10.1097/01.AOG.0000154161.03418.e3. PMC 1847590. PMID 15738025.
- ↑ "Minority Women's Health". Women's Health.gov.
- ↑ 80.0 80.1 80.2 "Black Women and High Prevalence of Fibroids". Fibroid Treatment Collective. November 29, 2010. Retrieved 30 March 2011.
- ↑ Wise LA, Radin RG, Palmer JR, Kumanyika SK, Rosenberg L (2010). "A prospective study of dairy intake and risk of uterine leiomyomata". Am. J. Epidemiol. 171 (2): 221–32. doi:10.1093/aje/kwp355. PMC 2800240. PMID 19955473. Lay summary – Science Daily.
- ↑ Wise LA, Palmer JR, Reich D, Cozier YC, Rosenberg L (March 2012). "Hair relaxer use and risk of uterine leiomyomata in African-American women". Am. J. Epidemiol. 175 (5): 432–40. doi:10.1093/aje/kwr351. PMC 3282879. PMID 22234483.
- ↑ http://officeofbudget.od.nih.gov/pdfs/FY11/Approp.%20History%20by%20IC%20(FINAL).pdf[]
- ↑ Bäcklin BM, Eriksson L, Olovsson M (March 2003). "Histology of uterine leiomyoma and occurrence in relation to reproductive activity in the Baltic gray seal (Halichoerus grypus)". Vet. Pathol. 40 (2): 175–80. doi:10.1354/vp.40-2-175. PMID 12637757.
External links
| Wikimedia Commons has media related to Uterine fibroids. |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
