Urocanase
Urocanase[1] (also known as imidazolonepropionate hydrolase or urocanate hydratase) is the enzyme that catalyzes the second step in the degradation of histidine, the hydration of urocanate into imidazolonepropionate.
- urocanate + H2O
 4,5-dihydro-4-oxo-5-imidazolepropanoate
4,5-dihydro-4-oxo-5-imidazolepropanoate
Inherited deficiency of urocanase leads to elevated levels of urocanic acid in the urine, a condition known as urocanic aciduria.
Urocanase is found in some bacteria (gene hutU), in the liver of many vertebrates and has also been found in the plant Trifolium repens (white clover). Urocanase is a protein of about 60 Kd, it binds tightly to NAD+ and uses it as an electrophil cofactor. A conserved cysteine has been found to be important for the catalytic mechanism and could be involved in the binding of the NAD+.
References
External links
|
|---|
| | 4.2.1: Hydro-Lyases | |
|---|
| | 4.2.2: Acting on polysaccharides | |
|---|
| | 4.2.3: Acting on phosphates | |
|---|
| | 4.2.99: Other | |
|---|
|
- Biochemistry overview
- Enzymes overview
- By EC number: 1.1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 10
- 11
- 13
- 14
- 15-18
- 2.1
- 3.1
- 4.1
- 5.1
- 6.1-3
|
|
|
|
|
|---|
| | | | K→acetyl-CoA | |
|---|
| | G | |
|---|
| |
|---|
| | Description |
- Metabolism
- Enzymes and pathways: citric acid cycle
- pentose phosphate
- glycoproteins
- glycosaminoglycans
- phospholipid
- cholesterol and steroid
- sphingolipids
- eicosanoids
- amino acid
- urea cycle
- nucleotide
|
|---|
| | Disorders |
- Citric acid cycle and electron transport chain
- Glycoprotein
- Proteoglycan
- Fatty-acid
- Phospholipid
- Cholesterol and steroid
- Eicosanoid
- Amino acid
- Purine-pyrimidine
- Heme metabolism
- Symptoms and signs
|
|---|
| | Treatment | |
|---|
|
|
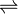 4,5-dihydro-4-oxo-5-imidazolepropanoate
4,5-dihydro-4-oxo-5-imidazolepropanoate 
