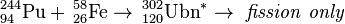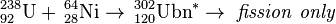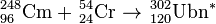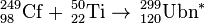Unbinilium
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol | unbinilium, Ubn | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | /uːnbaɪˈnɪliəm/ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative names | element 120, eka-radium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unbinilium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number | 120 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [320] (predicted)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | unknown, but probably an alkaline earth metal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, block | group 2 (alkaline earth metals), s-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Uuo] 8s2 (predicted)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| per shell | 2, 8, 18, 32, 32, 18, 8, 2 (predicted) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid (predicted)[2][3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 953 K (680 °C, 1256 °F) (predicted)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1973 K (1700 °C, 3092 °F) (predicted)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density near r.t. | 7 g·cm−3 (predicted)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 8.03–8.58 kJ·mol−1 (extrapolated)[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 1,[4] 2, 4 (predicted)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
1st: 578.9 kJ·mol−1 (predicted)[2] 2nd: 895.4–918.5 kJ·mol−1 (extrapolated)[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 200 pm (predicted)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 206–210 pm (extrapolated)[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 54143-58-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | IUPAC systematic element name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Unbinilium /uːnbaɪˈnɪliəm/, or eka-radium or element 120, is the temporary, systematic element name of a hypothetical chemical element in the periodic table with the temporary symbol Ubn and the atomic number 120. Since unbinilium should be an alkaline earth metal, it may have properties similar to radium or barium.
Attempts to date to synthesize the element using fusion reactions at low excitation energy have met with failure, though there are reports that the fission of unbinilium nuclei at very high excitation has been successfully measured, indicating a strong shell effect at Z=120.
Attempts at synthesis
Neutron evaporation
Following the success in obtaining ununoctium by the reaction between 249Cf and 48Ca, the discoverers started similar experiments in hope of creating unbinilium (element 120) from nuclei of 58Fe and 244Pu.[7] Isotopes of unbinilium are predicted to have alpha decay half-lives of the order of microseconds.[8][9] In March–April 2007, the synthesis of unbinilium was attempted at the Flerov Laboratory of Nuclear Reactions in Dubna by bombarding a plutonium-244 target with iron-58 ions.[10] Initial analysis revealed that no atoms of element 120 were produced providing a limit of 400 fb for the cross section at the energy studied.[11]
The Russian team are planning to upgrade their facilities before attempting the reaction again.[5]
In April 2007, the team at GSI attempted to create unbinilium using uranium-238 and nickel-64:[5]
No atoms were detected providing a limit of 1.6 pb on the cross section at the energy provided. The GSI repeated the experiment with higher sensitivity in three separate runs from April–May 2007, Jan–March 2008, and Sept–Oct 2008, all with negative results and providing a cross section limit of 90 fb.[5]
In June–July 2010, scientists at the GSI attempted the fusion reaction:[5]
They were unable to detect any atoms, but exact details are not currently available.[5]
In August–October 2011, a different team at the GSI using the TASCA facility tried the new reaction:[5]
Results from this experiment are not yet available.[5]
Compound nucleus fission
Unbinilium is of interest because it is part of the hypothesized island of stability, with the compound nucleus 302Ubn being the most stable of those that can be created directly by current methods. It has been calculated that Z=120 may in fact be the next proton magic number, rather than at Z=114 or 126.
Several experiments have been performed between 2000–2008 at the Flerov laboratory of Nuclear Reactions in Dubna studying the fission characteristics of the compound nucleus 302Ubn. Two nuclear reactions have been used, namely 244Pu+58Fe and 238U+64Ni. The results have revealed how nuclei such as this fission predominantly by expelling closed shell nuclei such as 132Sn (Z=50, N=82). It was also found that the yield for the fusion-fission pathway was similar between 48Ca and 58Fe projectiles, indicating a possible future use of 58Fe projectiles in superheavy element formation.[12]
In 2008, the team at GANIL, France, described the results from a new technique which attempts to measure the fission half-life of a compound nucleus at high excitation energy, since the yields are significantly higher than from neutron evaporation channels. It is also a useful method for probing the effects of shell closures on the survivability of compound nuclei in the super-heavy region, which can indicate the exact position of the next proton shell (Z=114, 120, 124, or 126). The team studied the nuclear fusion reaction between uranium ions and a target of natural nickel:
The results indicated that nuclei of unbinilium were produced at high (~70 MeV) excitation energy which underwent fission with measurable half-lives > 10−18 s. Although very short, the ability to measure such a process indicates a strong shell effect at Z=120. At lower excitation energy (see neutron evaporation), the effect of the shell will be enhanced and ground-state nuclei can be expected to have relatively long half-lives. This result could partially explain the relatively long half-life of 294Uuo measured in experiments at Dubna. Similar experiments have indicated a similar phenomenon at Z=124 (see unbiquadium) but not for flerovium, suggesting that the next proton shell does in fact lie at Z>120.[13][14]
Future reactions
The team at RIKEN have begun a program utilizing 248Cm targets and have indicated future experiments to probe the possibility of Z=120 being the next magic number using the aforementioned nuclear reactions to form 302Ubn.[15]
Calculated decay characteristics
In a quantum tunneling model with mass estimates from a macroscopic-microscopic model, the alpha-decay half-lives of several unbinilium isotopes (292–304Ubn) have been predicted to be around 1–20 microseconds.[16][17][18][19]
Extrapolated chemical properties
Unbinilium should be highly reactive, according to periodic trends, as this element is in the same periodic table column as the alkaline earth metals. It would be much more reactive than any other lighter elements of this group. If group reactivity is followed, this element would react violently in air to form an oxide (UbnO), in water to form the hydroxide, which would be a strong base and highly explosive in terms of flammability, and with the halogens to form salts (such as UbnCl2).[20]
Although unbinilium is expected to behave typically for an alkaline earth metal, showing a strong +2 oxidation state, the energetic properties of its valence electrons would increase its ionization energies; hence, unbinilium may have a lower metallic and ionic radius than expected, and may behave more similarly to calcium and strontium than barium or radium.[21] Unbinilium is also predicted to be the first alkaline earth metal to display the +4 oxidation state, due to the ionization energy of the 7p3/2 electrons, which is predicted to be very low.[2] The +1 state may also be stable in isolation.[4]
Target-projectile combinations leading to Z=120 compound nuclei
The below table contains various combinations of targets and projectiles which could be used to form compound nuclei with an atomic number of 120.
| Target | Projectile | CN | Attempt result |
|---|---|---|---|
| 208Pb | 88Sr | 296Ubn | Reaction yet to be attempted[2] |
| 238U | 64Ni | 302Ubn | Failure to date, σ < 94 fb |
| 244Pu | 58Fe | 302Ubn | Failure to date, σ < 0.4 pb |
| 248Cm | 54Cr | 302Ubn | Failure to date, not all details available |
| 250Cm | 54Cr | 304Ubn | Reaction yet to be attempted |
| 249Cf | 50Ti | 299Ubn | Results are not yet available |
| 252Cf | 50Ti | 302Ubn | Reaction yet to be attempted |
| 257Fm | 48Ca | 305Ubn | Reaction yet to be attempted |
Theoretical calculations on evaporation cross sections
The below table contains various targets-projectile combinations for which calculations have provided estimates for cross section yields from various neutron evaporation channels. The channel with the highest expected yield is given.
MD = multi-dimensional; DNS = dinuclear system; AS = advanced statistical; σ = cross section
| Target | Projectile | CN | Channel (product) | ~σmax | Model | Ref |
|---|---|---|---|---|---|---|
| 208Pb | 88Sr | 296Ubn | 1n (295Ubn) | 70 fb | DNS | [22] |
| 208Pb | 87Sr | 295Ubn | 1n (294Ubn) | 80 fb | DNS | [22] |
| 208Pb | 88Sr | 296Ubn | 1n (295Ubn) | <0.1 fb | MD | [23] |
| 238U | 64Ni | 302Ubn | 3n (299Ubn) | 3 fb | MD | [23] |
| 238U | 64Ni | 302Ubn | 2n (300Ubn) | 0.5 fb | DNS | [24] |
| 238U | 64Ni | 302Ubn | 4n (298Ubn) | 2 ab | DNS-AS | [25] |
| 244Pu | 58Fe | 302Ubn | 4n (298Ubn) | 5 fb | MD | [23] |
| 244Pu | 58Fe | 302Ubn | 3n (299Ubn) | 8 fb | DNS-AS | [25] |
| 248Cm | 54Cr | 302Ubn | 3n (299Ubn) | 10 pb | DNS-AS | [25] |
| 248Cm | 54Cr | 302Ubn | 4n (298Ubn) | 30 fb | MD | [23] |
| 249Cf | 50Ti | 299Ubn | 4n (295Ubn) | 45 fb | MD | [23] |
| 249Cf | 50Ti | 299Ubn | 3n (296Ubn) | 40 fb | MD | [23] |
| 257Fm | 48Ca | 305Ubn | 3n (302Ubn) | 70 fb | DNS | [24] |
See also
- Island of stability: flerovium–unbinilium–unbihexium
- Radium
- Barium
References
- ↑ 1.0 1.1 Fricke, B.; Waber, J. T. (1971). "Theoretical Predictions of the Chemistry of Superheavy Elements" (PDF). Actinides Reviews 1: 433–485. Retrieved 7 August 2013.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Haire, Richard G. (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean. The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN 1-4020-3555-1.
- ↑ 3.0 3.1 3.2 3.3 Bonchev, Danail; Kamenska, Verginia (1981). "Predicting the properties of the 113-120 transactinide elements". Journal of Physical Chemistry (American Chemical Society) 85 (9): 1177–1186. doi:10.1021/j150609a021.
- ↑ 4.0 4.1 Thayer, John S. (2010). Relativistic Effects and the Chemistry of the Heavier Main Group Elements. p. 84. doi:10.1007/978-1-4020-9975-5_2.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 Dullman, C.E. Superheavy Element Research Superheavy Element - News from GSI and Mainz. University Mainz
- ↑ 6.0 6.1 6.2 Hofmann, Sigurd (2013). Overview and Perspectives of SHE Research at GSI SHIP. p. 23–32. doi:10.1007/978-3-319-00047-3.
- ↑ "A New Block on the Periodic Table" (PDF). Lawrence Livermore National Laboratory. April 2007. Retrieved 2008-01-18.
- ↑ Chowdhury, P. Roy; Samanta, C.; Basu, D. N. (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Physical Reviews C 77 (4): 044603. arXiv:0802.3837. Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603.
- ↑ Chowdhury, R. P.; Samanta, C.; Basu, D.N. (2008). "Nuclear half-lives for α -radioactivity of elements with 100 ≤ Z ≤ 130". At. Data & Nucl. Data Tables 94 (6): 781–806. arXiv:0802.4161. Bibcode:2008ADNDT..94..781C. doi:10.1016/j.adt.2008.01.003.
- ↑ Synthesis of New Nuclei and Study of Nuclear Properties and Heavy-Ion Reaction Mechanisms. jinr.ru
- ↑ Oganessian, Yu.Ts.; Utyonkov, V.; Lobanov, Yu.; Abdullin, F.; Polyakov, A.; Sagaidak, R.; Shirokovsky, I.; Tsyganov, Yu. et al. (2009). "Attempt to produce element 120 in the 244Pu+58Fe reaction". Phys. Rev. C 79 (2): 024603. Bibcode:2009PhRvC..79b4603O. doi:10.1103/PhysRevC.79.024603.
- ↑ see Flerov lab annual reports 2000–2004
- ↑ Natowitz, Joseph (2008). "How stable are the heaviest nuclei?". Physics 1: 12. Bibcode:2008PhyOJ...1...12N. doi:10.1103/Physics.1.12.
- ↑ Morjean, M.; Jacquet, D.; Charvet, J.; l’Hoir, A.; Laget, M.; Parlog, M.; Chbihi, A.; Chevallier, M. et al. (2008). "Fission Time Measurements: A New Probe into Superheavy Element Stability". Phys. Rev. Lett. 101 (7): 072701. Bibcode:2008PhRvL.101g2701M. doi:10.1103/PhysRevLett.101.072701. PMID 18764526.
- ↑ see slide 11 in Future Plan of the Experimental Program on Synthesizing the Heaviest Element at RIKEN
- ↑ P. Roy Chowdhury; C. Samanta & D. N. Basu (2006). "α decay half-lives of new superheavy elements". Phys. Rev. C 73: 014612. arXiv:nucl-th/0507054. Bibcode:2006PhRvC..73a4612C. doi:10.1103/PhysRevC.73.014612.
- ↑ Samanta, C.; Chowdhury, P. Roy & Basu, D.N. (2007). "Predictions of alpha decay half lives of heavy and superheavy elements". Nucl. Phys. A 789: 142–154. arXiv:nucl-th/0703086. Bibcode:2007NuPhA.789..142S. doi:10.1016/j.nuclphysa.2007.04.001.
- ↑ Chowdhury, P. Roy; Samanta, C. & Basu, D. N. (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Phys. Rev. C 77 (4): 044603. arXiv:0802.3837. Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603.
- ↑ Chowdhury, P. Roy; Samanta, C. & Basu, D. N. (2008). "Nuclear half-lives for α-radioactivity of elements with 100 ≤ Z ≤ 130". At. Data & Nucl. Data Tables 94 (6): 781–806. arXiv:0802.4161. Bibcode:2008ADNDT..94..781C. doi:10.1016/j.adt.2008.01.003.
- ↑ Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements (New ed.). New York, NY: Oxford University Press. p. 586. ISBN 978-0-19-960563-7.
- ↑ Seaborg (c. 2006). "transuranium element (chemical element)". Encyclopædia Britannica. Retrieved 2010-03-16.
- ↑ 22.0 22.1 Feng, Zhao-Qing; Jin, Gen-Ming; Li, Jun-Qing; Scheid, Werner (2007). "Formation of superheavy nuclei in cold fusion reactions". Physical Review C 76 (4): 044606. arXiv:0707.2588. Bibcode:2007PhRvC..76d4606F. doi:10.1103/PhysRevC.76.044606.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 Zagebraev, V; Greiner, W (2008). "Synthesis of superheavy nuclei: A search for new production reactions". Physical Review C 78 (3): 034610. arXiv:0807.2537. Bibcode:2008PhRvC..78c4610Z. doi:10.1103/PhysRevC.78.034610.
- ↑ 24.0 24.1 Feng, Z; Jin, G; Li, J; Scheid, W (2009). "Production of heavy and superheavy nuclei in massive fusion reactions". Nuclear Physics A 816: 33. arXiv:0803.1117. Bibcode:2009NuPhA.816...33F. doi:10.1016/j.nuclphysa.2008.11.003.
- ↑ 25.0 25.1 25.2 Nasirov, A. K.; Giardina, G.; Mandaglio, G.; Manganaro, M.; Hanappe, F.; Heinz, S.; Hofmann, S.; Muminov, A. et al. (2009). "Quasifission and fusion-fission in reactions with massive nuclei: Comparison of reactions leading to the Z=120 element". Physical Review C 79 (2): 024606. arXiv:0812.4410. Bibcode:2009PhRvC..79b4606N. doi:10.1103/PhysRevC.79.024606.
| Extended periodic table (Large version) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||||||||||||||||
| 1 | H | He | ||||||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | 113 | Fl | 115 | Lv | 117 | 118 | ||||
| 8 | 119 | 120 | |
141 | 142 | 143 | 144 | 145 | 146 | 147 | 148 | 149 | 150 | 151 | 152 | 153 | 154 | 155 | 156 | 157 | 158 | 159 | 160 | 161 | 162 | 163 | 164 | |||||||||
| 9 | 165 | 166 | 167 | 168 | 169 | 170 | 171 | 172 | ||||||||||||||||||||||||||||
| 10 | | |||||||||||||||||||||||||||||||||||
| |
121 | 122 | 123 | 124 | 125 | 126 | 127 | 128 | 129 | 130 | 131 | 132 | 133 | 134 | 135 | 136 | 137 | 138 | 139 | 140 | ||||||||||||||||
| |
| |||||||||||||||||||||||||||||||||||
| Legend |