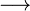Umbelliferone
 | |
| Names | |
|---|---|
| IUPAC name
7-Hydroxychromen-2-one | |
| Other names
7-hydroxycoumarin, hydrangine, skimmetine, beta-umbelliferone | |
| Identifiers | |
| 93-35-6 | |
| ChEBI | CHEBI:27510 |
| ChEMBL | ChEMBL51628 |
| ChemSpider | 4444774 |
| |
| Jmol-3D images | Image |
| PubChem | 5281426 |
| |
| Properties | |
| C9H6O3 | |
| Molar mass | 162.14 g/mol |
| Melting point | 230 °C (446 °F; 503 K) (decomposes) |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and beta-umbelliferone, is a widespread natural product of the coumarin family.
It absorbs ultraviolet light strongly at several wavelengths. Despite several indications that this chemical is photomutagenic, it is used in sunscreens.[1] Umbelliferone has been reported to have antioxidant properties.[2]
It is a yellowish-white crystalline solid that has a slight solubility in hot water, but high solubility in ethanol.
Natural occurrences and name
Umbelliferone's name is from the umbelliferae family of plants, and the plant family in turn was named for their umbrella-shaped inflorescences, each called an umbel.
Umbelliferone occurs in many familiar plants from the Apiaceae (Umbelliferae) family such as carrot, coriander and garden angelica, as well as in plants from other families, such as the mouse-ear hawkweed (Hieracium pilosella, Asteraceae) or the bigleaf hydrangea (Hydrangea macrophylla, Hydrangeaceae, under the name hydrangine).
It is one of the components of asafoetida, the dried latex from the giant fennel (Ferula communis).
It is also found in Justicia pectoralis (Acanthaceae).[3][4]
Biosynthesis
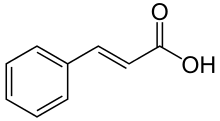
Umbelliferone is a phenylpropanoid and as such is synthesized from L-phenylalanine, which in turn is produced via the shikimate pathway. Phenylalanine is lysated into cinnamic acid, followed by hydroxylation by cinnamate 4-hydroxylase to yield 4-coumaric acid. The 4-coumaric acid is again hydroxylated by cinnamate/coumarate 2-hydroxylase to yield 2,4-dihydroxy-cinnamic acid (umbellic acid) followed by a bond rotation of the unsaturated bond adjacent to the carboxylic acid group. Finally an intramolecular attack from the hydroxyl group of C2' to the carboxylic acid group closes the ring and forms the lactone umbelliferone.
Chemical synthesis
Umbelliferone is traditionally synthesized using the Pechmann condensation, from resorcinol and formylacetic acid (generated from malic acid in situ).
A newer synthesis uses methyl propionate and a palladium catalyst.
Ultraviolet fluorescence
Umbelliferone absorbs strongly at 300, 305 and 325 nm, with log ε values of 3.9, 3.95 and 4.15 respectively, and it fluoresces blue in both ultraviolet and visible light. The powerful absorption at three different wavelengths, coupled with the fact that the energy is dissipated safely as visible light, make umbelliferone a useful sunscreen agent. The absorption changes in alkaline solution, since the phenolic hydroxyl group is deprotonated (pKa = 7.7).
Uses
The ultraviolet activity of umbelliferone led to its use as a sunscreen agent, and an optical brightener for textiles. It has also been used as a gain medium for dye lasers. Umbelliferone can be used as a fluorescence indicator for metal ions such as copper and calcium. It acts as a pH indicator in the range 6.5-8.9
Derivatives of umbelliferone
Umbelliferone is the parent compound for a large number of natural products. Herniarin (7-O-methylumbelliferone or 7-methoxycoumarin) occurs in the leaves of water hemp (Eupatorium ayapana) and rupturewort (Herniaria). O-Glycosylated derivatives such as skimmin (7-O-β-D-glucopyranosylumbelliferone) occur naturally and are used for the fluorimetric determination of glycoside hydrolase enzymes. Isoprenylated derivatives are also widespread, such as marmin (found in grapefruit skin and in the bark of the Bael tree) and furocoumarins such as marmesin, angelicin, and psoralen.
 Herniarin and marmin, umbelliferone derivatives
Herniarin and marmin, umbelliferone derivatives
Umbelliferone 7-apiosylglucoside can be isolated from the root of Gmelina arborea.[5]
See also
References
- ↑ Du, Lupei (2008). "Rational design of a fluorescent hydrogen peroxide probe based on the umbelliferone fluorophore". Tetrahedron Letters 49 (19): 3045–3048. doi:10.1016/j.tetlet.2008.03.063.
- ↑ "UMBELLIFERONE". www.chemicalland21.com. Retrieved 21 November 2011.
- ↑ Leal, L. K. A. M.; A. A. G. Ferreira; G. A. Bezerra; F. J. A. Matos; G. S. B. Viana (May 2000). "Antinociceptive, anti-inflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: a comparative study". Journal of Ethnopharmacology 70 (2): 151–159. doi:10.1016/S0378-8741(99)00165-8. ISSN 0378-8741. PMID 10771205. Retrieved 2010-06-26.
- ↑ Lino, C. S.; M. L. Taveira; G. S. B. Viana; F. J. A. Matos (1997). "Analgesic and antiinflammatory activities of Justicia pectoralis Jacq and its main constituents: coumarin and umbelliferone". Phytotherapy Research 11 (3): 211–215. doi:10.1002/(SICI)1099-1573(199705)11:3<211::AID-PTR72>3.0.CO;2-W. Retrieved 2010-06-26.
- ↑ P. Satyanarayana, P. Subrahmanyam, R. Kasai and O. Tanaka (1985). "An apiose-containing coumarin glycoside from gmelina arborea root". Phytochemistry 24 (8): 1862–1863. doi:10.1016/S0031-9422(00)82575-3.
Further reading
- Dean, F.M. (1963). Naturally Occurring Oxygen Ring Compounds. London: Butterworths. ISBN 978-0-408-26750-2.
- Joule, J.A.; Mills, K. (2000). Heterocyclic Chemistry (4th ed.). Oxford: Blackwell Science. ISBN 978-0-632-05453-4.
- Barton, D.H.R.; Nakanishi, K.; Meth-Cohn, O., eds. (1999). Comprehensive Natural Products Chemistry 2. Oxford: Elsevier. p. 677. ISBN 978-0-08-043154-3.
External links
| ||||||||||||||||||||||||||||||||||||||