Tunicate
| Tunicate Temporal range: Cambrian "Stage 3"–recent[1] | |
|---|---|
 | |
| Sea tulips (Pyura spinifera) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Subphylum: | Tunicata Lamarck 1816[2][3] |
| Classes[2][4] | |
| Synonyms | |
|
Urochordata Lankester 1877 | |
A tunicate is a marine invertebrate animal, a member of the subphylum Tunicata, which is part of the Chordata, a phylum which includes all animals with dorsal nerve cords and notochords. The subphylum was at one time called Urochordata, and the term urochordates is still sometimes used for these animals. Some tunicates live as solitary individuals, but others replicate by budding and become colonies, each unit being known as a zooid. They are marine filter feeders with a water-filled, sac-like body structure and two tubular openings, known as siphons, through which they draw in and expel water. During their respiration and feeding, they take in water through the incurrent (or inhalant) siphon and expel the filtered water through the excurrent (or exhalant) siphon. Most adult tunicates are sessile and are permanently attached to rocks or other hard surfaces on the ocean floor; others, such as salps, doliolids and pyrosomes, swim in the pelagic zone of the sea as adults. Various species are commonly known as sea squirts, sea pork, sea livers, or sea tulips.
The Tunicata first appear in the fossil record in the early Cambrian period. Despite their simple appearance and very different adult form, their close relationship to the vertebrates is shown by the fact that during their mobile larval stage, they possess a notochord or stiffening rod and resemble a tadpole. Their name derives from their unique outer covering or "tunic" which is formed from proteins and carbohydrates and acts as an exoskeleton. In some species, it is thin, translucent, and gelatinous, while in others it is thick, tough, and stiff.
Taxonomy


About 2,150 species of tunicates occur in the world's oceans, mostly living in shallow water. The most numerous group is the ascidians, and fewer than 100 species of these are found at depths greater than 200 m (660 ft).[5] Some are solitary animals leading a sessile existence attached to the seabed, but others are colonial and a few are pelagic. Some are supported by a stalk, but most are attached directly to the substrate, which may be a rock, shell, coral, seaweed, mangrove root, dock, piling, or ship's hull. They are found in a range of solid or translucent colours and may resemble seeds, grapes, peaches, barrels, or bottles. One of the largest is a stalked sea tulip, Pyura pachydermatina, which can grow to be over a metre (yard) tall.[5]
The Tunicata were established by Jean-Baptiste Lamarck in 1816. In 1881, Francis Maitland Balfour introduced a second name for the same group, "Urochorda", to emphasize the affinity of the group to other chordates.[6] No doubt largely because of his influence, various authors supported the term, either as such, or as "Urochordata", but the usage is invalid because "Tunicata" has precedence and grounds for superseding the name never existed. Accordingly, the current (formally correct) trend is to abandon the name Urochorda or Urochordata in favour of the original Tunicata, and the name Tunicata is almost invariably used in modern scientific works. It is accepted as valid by the World Register of Marine Species[7] and by the Integrated Taxonomic Information System.[8]
Various common names are used for different species. Sea tulips are tunicates with colourful bodies supported on slender stalks.[9] Sea squirts are so named because of their habit of contracting their bodies sharply and squirting out water when disturbed.[10] Sea liver and sea pork get their names from the resemblance of their dead colonies to pieces of meat.[11]
Classification
Tunicates are more closely related to craniates (including hagfish, lampreys, and jawed vertebrates) than to lancelets, echinoderms, hemichordates, Xenoturbella or other invertebrates.[12][13][14]
The clade comprising tunicates and vertebrates is called Olfactores.[15]
The Tunicata contain about 2,150 described species,[5] traditionally divided into these classes:
- Ascidiacea (Aplousobranchia, Phlebobranchia, and Stolidobranchia)
- Thaliacea (Pyrosomida, Doliolida, and Salpida)
- Appendicularia (Larvacea)
Members of the Sorberacea were included in Ascidiacea in 2011 as a result of rDNA sequencing studies.[4] Although the traditional classification is provisionally accepted, newer evidence suggests the Ascidiacea are an artificial group of paraphyletic status.[16][17]
Fossil record

Undisputed fossils of tunicates are rare. The best known and earliest unequivocally identified species is Shankouclava shankouense from the Lower Cambrian Maotianshan Shale at Shankou village, Anning, near Kunming (South China).[18] There is also a common bioimmuration, (Catellocaula vallata), of a possible tunicate found in Upper Ordovician bryozoan skeletons of the upper midwestern United States.[19]
Three enigmatic species also were found from the Ediacaran period – Ausia fenestrata from the Nama Group of Namibia, the sac-like Yarnemia acidiformis, and a second new Ausia-like genus from the Onega Peninsula, White Sea, of northern Russia, Burykhia hunti. Results of a new study have shown possible affinity of these Ediacaran organisms to the ascidians.[20][21] Ausia and Buryhia lived in shallow coastal waters, slightly more than 555–548 million years ago and are believed to be the oldest evidence of the chordate lineage of metazoans.[21] The Russian Precambrian fossil Yarnemia is tentatively identified as a tunicate, though, because its fossils are nowhere near as well-preserved as those of Ausia and Burykhia, this identification is questioned.
Fossils of tunicates are rare because their bodies decay soon after death, but in some tunicate families, microscopic spicules are present which may be preserved as microfossils. These spicules have occasionally been found in Jurassic and later rocks, but as few palaeontologists are familiar with them, they may have been mistaken for sponge spicules.[22]
Hybridization studies
A multitaxon molecular study in 2010 looked for evidence that sea squirts are descended from a hybrid between a chordate and a protostome ancestor, and for evidence of sea urchin-tunicate hybridization in the genes of modern sea squirts. It found no evidence for either hypothesis.[23][24]
Anatomy
Body form

Colonies of tunicates occur in a range of forms and in the degree to which individual organisms, known as zooids, integrate with one another. In the simplest systems, the individual animals are widely separated, but linked together by horizontal connections called stolons, which grow across the seabed. Other species have the zooids growing closer together in a tuft or clustered together and sharing a common base. The most advanced colonies involve the integration of the zooids into a common structure surrounded by the tunic. These may have separate buccal siphons and a single central atrial siphon and may be organized into larger systems with hundreds of star-shaped units. Often the zooids in a colony are tiny, but very numerous, and the colonies can form large encrusting or mat-like patches.[5]
Body structure
By far the largest class of tunicates is the Ascidiacea. The body of an ascidiacean is surrounded by a test or tunic, from which the subphylum derives its name. This varies in thickness between species but may be tough, resembling cartilage, thin and delicate, or transparent and gelatinous. The tunic is composed of proteins and complex carbohydrates, and includes tunicin, a variety of cellulose. The tunic is unique among invertebrate exoskeletons in that it can grow as the animal enlarges and does not need to be periodically shed. Inside the tunic is the body wall or mantle composed of connective tissue, muscle fibres, blood vessels, and nerves. Two openings are found in the body wall, the buccal siphon at the top through which water flows into the interior and the atrial siphon on the ventral side through which it is expelled. A large pharynx occupies most of the interior of the body. It is a muscular tube linking the buccal opening with the rest of the gut. It has a ciliated groove known as an endostyle on its ventral surface, and this secretes a mucous net which collects food particles and is wound up on the dorsal side of the pharynx. The gullet, at the lower end of the pharynx, links it to a loop of gut which terminates near the atrial siphon. The walls of the pharynx are perforated by several bands of slits, known as stigmata, through which water escapes into the surrounding water-filled cavity, the atrium. This is criss-crossed by various rope-like mesenteries which extend from the mantle and provide support for the pharynx, preventing it from collapsing, and also hold up the other organs.[5]
The Thaliacea, the other main class of tunicates, are characterised by free-swimming, pelagic individuals. They are all filter feeders using a pharyngeal mucous net to catch their prey. The pyrosomes are bioluminous colonial tunicates with a hollow cylindrical structure. The buccal siphons are on the outside and the atrial siphons inside. About 10 species are known, and all are found in the tropics. The 23 species of doliolids are small, mostly under 2 cm (0.79 in) long. They are solitary, have the two siphons at opposite ends of their barrel-shaped bodies, and swim by jet propulsion. The 40 species of salps are also small, under 4 cm (1.6 in) long, and found in the surface waters of both warm and cold seas. They also move by jet propulsion, and often form long chains by budding off new individuals.[5]
A third class, the Larvacea (or Appendicularia), is the only group of tunicates to retain their chordate characteristics in the adult state by neoteny. The 70 species of larvaceans superficially resemble the tadpole larvae of amphibians, although the tail is at right angles to the body. The notochord is retained, and the animals, mostly under 1 cm long, are propelled by undulations of the tail. They secrete an external mucous net known as a house, which may completely surround them and is very efficient at trapping planktonic particles.[5]
Physiology and internal anatomy

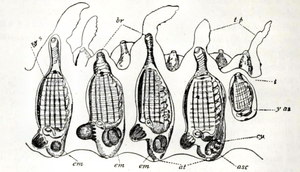
Like other chordates, tunicates have a notochord during their early development, but by the time they have completed their larval stages, they have lost all myomeric segmentation throughout the body. As members of the Chordata, they are true Coelomata with endoderm, ectoderm, and mesoderm, but they do not develop very clear coelomic body cavities, if any at all. Whether they do or not, by the end of their larval development, all that remain are the pericardial, renal, and gonadal cavities of the adults. Except for the heart, gonads, and pharynx (or branchial sac), the organs are enclosed in a membrane called an epicardium, which is surrounded by the jelly-like mesenchyme. Tunicates begin life in a mobile larval stage that resembles a tadpole. A minority of species, those in the Larvacea, retain the general larval form throughout life, but most Tunicata very rapidly settle down and attach themselves to a suitable surface, later developing into a barrel-like and usually sedentary adult form. The Thaliacea, however, are pelagic throughout their lives and may have complex lifecycles.[25]
Tunicates have a well-developed heart and circulatory system. The heart is a double U-shaped tube situated just below the gut. The blood vessels are simple connective tissue tubes, and their blood has several types of corpuscle. The blood may appear pale green, but this is not due to any respiratory pigments, and oxygen is transported dissolved in the plasma. Exact details of the circulatory system are unclear, but the gut, pharynx, gills, gonads, and nervous system seem to be arranged in series rather than in parallel, as happens in most other animals. Every few minutes, the heart stops beating and then restarts, pumping fluid in the reverse direction.[5]
Tunicate blood has some unusual features. In some species of Ascidiidae and Perophoridae, it contains high concentrations of the transitional metal vanadium and vanadium-associated proteins in vacuoles in blood cells known as vanadocytes. Some tunicates can concentrate vanadium up to a level ten million times that of the surrounding seawater. It is stored in a +3 oxidation form that requires a pH of less than 2 for stability, and this is achieved by the vacuoles also containing sulphuric acid. The vanadocytes are later deposited just below the outer surface of the tunic, where their presence is thought to deter predation. Other species of tunicates concentrate lithium, iron, niobium, and tantalum, which may serve a similar function.[5]
_cophocerca_001.png)
Tunicates lack the kidney-like metanephridial organs typical of deuterostomes. Most have no excretory structures, but rely on the diffusion of ammonia across their tissues to rid themselves of nitrogenous waste, though some have a simple excretory system. The typical renal organ is a mass of large clear-walled vesicles that occupy the rectal loop, and the structure has no duct. Each vesicle is a remnant of a part of the primitive coelom, and its cells extract nitrogenous waste matter from circulating blood. They accumulate the wastes inside the vesicles as urate crystals, and do not have any obvious means of disposing of the material during their lifetimes.[25]
Adult tunicates have a hollow cerebral ganglion, equivalent to a brain, and a hollow structure known as a neural gland. Both originate from the embryonic neural tube and are located between the two siphons. Nerves arise from the two ends of the ganglion; those from the anterior end innervate the buccal siphon and those from the posterior end supply the rest of the body, the atrial siphon, organs, gut and the musculature of the body wall. There are no sense organs but there are sensory cells on the siphons, the buccal tentacles and in the atrium.[5]
Tunicates are unusual among animals in that they produce a large fraction of their tunic and some other structures in the form of cellulose. The production in animals of cellulose is so unusual, at first some researchers denied its presence outside plants, but for some time now it has been accepted that it occurs in the dermis of mammals. However, the tunicates are unique in their scale of application and production of the material, and possibly in having a functional cellulose synthesising enzyme.[26] When, in 1845, Carl Schmidt first announced the presence in the test of some ascidians of a substance very similar to cellulose, he called it "tunicine", but it now is recognised as cellulose rather than any alternative substance.[27][28][29]
Feeding

Nearly all tunicates are suspension feeders, capturing planktonic particles by filtering sea water through their bodies. Ascidians are typical in their digestive processes, but other tunicates have similar systems. Water is drawn into the body through the buccal siphon by the action of cilia lining the gill slits. To obtain enough food, an average ascidian needs to process one body-volume of water per second.[5] This is drawn through a net lining the pharynx which is being continuously secreted by the endostyle. The net is made of sticky mucus threads with holes about 0.5 µm in diameter which can trap planktonic particles including bacteria. The net is rolled up on the dorsal side of the pharynx, and it and the trapped particles are drawn into the oesophagus. The gut is U-shaped and also ciliated to move the contents along. The stomach is an enlarged region at the lowest part of the U-bend. Here, digestive enzymes are secreted and a pyloric gland adds further secretions. After digestion, the food is moved on through the intestine, where absorption takes place, and the rectum, where undigested remains are formed into faecal pellets or strings. The anus opens into the dorsal or cloacal part of the peribranchial cavity near the atrial siphon. Here, the faeces are caught up by the constant stream of water which carries the waste to the exterior. The animal orientates itself to the current in such a way that the buccal siphon is always upstream and does not draw in contaminated water.[5]
Some ascidians that live on soft sediments are detritivores. A few deepwater species, such as Megalodicopia hians, are sit-and-wait predators, trapping tiny crustacea, nematodes, and other small invertebrates with the muscular lobes which surround their buccal siphons. Certain tropical species in the family Didemnidae have symbiotic green algae or cyanobacteria in their tunics, and one of these symbionts, Prochloron, is unique to tunicates. Excess photosynthetic products are assumed to be available to the host.[5]
Lifecycle

Ascidians are almost all hermaphrodites, and each has a single ovary and testis, either near the gut or on the body wall. In some solitary species, sperm and eggs are shed into the sea and the larvae are planktonic. In others, especially colonial species, sperm is released into the water and drawn into the atria of other individuals with the incoming water current. Fertilisation takes place here and the eggs are brooded through their early developmental stages.[25] Some larval forms appear very much like primitive chordates with a notochord (stiffening rod) and superficially resemble small tadpoles. These swim by undulations of the tail and may have a simple eye, an ocellus, and a balancing organ, a statocyst.[30]
When sufficiently developed, the larva of the sessile species finds a suitable rock and cements itself in place. The larval form is not capable of feeding, though it may have a rudimentary digestive system,[30] and is only a dispersal mechanism. Many physical changes occur to the tunicate's body during metamorphosis, one of the most significant being the reduction of the cerebral ganglion, which controls movement and is the equivalent of the human brain. From this comes the common saying that the sea squirt "eats its own brain".[31] In some classes, the adults remain pelagic (swimming or drifting in the open sea), although their larvae undergo similar metamorphoses to a higher or lower degree.[25] Colonial forms also increase the size of the colony by budding off new individuals to share the same tunic.[32]
Pyrosome colonies grow by budding off new zooids near the posterior end of the colony. Sexual reproduction starts within a zooid with an internally fertilised egg. This develops directly into an oozooid without any intervening larval form. This buds precociously to form four blastozooids which become detached in a single unit when the oozoid disintegrates. The atrial siphon of the oozoid becomes the exhalent siphon for the new, four-zooid colony.[5]

Doliolids have a very complex lifecycle that includes various zooids with different functions. The sexually reproducing members of the colony are known as gonozooids. Each one is a hermaphrodite with the eggs being fertilised by sperm from another individual. The gonozooid is viviparous, and at first, the developing embryo feeds on its yolk sac before being released into the sea as a free-swimming, tadpole-like larva. This undergoes metamorphosis in the water column into an oozooid. This is known as a "nurse" as it develops a tail of zooids produced by budding asexually. Some of these are known as trophozooids, have a nutritional function, and are arranged in lateral rows. Others are phorozooids, have a transport function, and are arranged in a single central row. Other zooids link to the phorozooids, which then detach themselves from the nurse. These zooids develop into gonozooids, and when these are mature, they separate from the phorozooids to live independently and start the cycle over again. Meanwhile, the phorozooids have served their purpose and disintegrate. The asexual phase in the lifecycle allows the doliolid to multiply very rapidly when conditions are favourable.[5]
Salps also have a complex lifecycle with an alternation of generations. In the solitary life history phase, an oozoid reproduces asexually, producing a chain of tens or hundreds of individual zooids by budding along the length of a stolon. The chain of salps is the 'aggregate' portion of the lifecycle. The aggregate individuals, known as blastozooids, remain attached together while swimming and feeding and growing larger. The blastozooids are sequential hermaphrodites. An egg in each is fertilized internally by a sperm from another colony. The egg develops in a brood sac inside the blastozooid and has a placental connection to the circulating blood of its "nurse". When it fills the blastozooid's body, it is released to start the independent life of an oozooid.[5]
Larvaceans only reproduce sexually. They are protandrous hermaphrodites, except for Oikopleura dioica which is gonochoric, and a larva resembles the tadpole larva of ascidians. Once the trunk is fully developed, the larva undergoes "tail shift", in which the tail moves from a rearward position to a ventral orientation and twists through 90° relative to the trunk. The larva consists of a small, fixed number of cells, and grows by enlargement of these rather than cell division. Development is very rapid and only takes seven hours for a zygote to develop into a house-building juvenile starting to feed.[5]
During embryonic development, tunicates exhibit determinate cleavage, where the fate of the cells is set early on with reduced cell numbers and genomes that are rapidly evolving. In contrast, the amphioxus and vertebrates show cell determination relatively late in development and cell cleavage is indeterminate. The genome evolution of amphioxus and vertebrates is also relatively slow.[33]
Promotion of out-crossing
Ciona intestinalis (class Ascidiacea) is a hermaphrodite that releases sperm and eggs into the surrounding seawater almost simultaneously. It is self-sterile, and thus has been used for studies on the mechanism of self-incompatibility.[34] Self/non-self-recognition molecules play a key role in the process of interaction between sperm and the vitelline coat of the egg. It appears that self/non-self recognition in ascidians such as C. intestinalis is mechanistically similar to self-incompatibility systems in flowering plants.[34] Self-incompatibility promotes out-crossing, and thus provides the adaptive advantage at each generation of the masking of deleterious recessive mutations (i.e. genetic complementation)[35] and the avoidance of inbreeding depression.
Botryllus schlosseri (class Ascidiacea) is a colonical tunicate, a member of the only group of chordates that are able to reproduce both sexually and asexually. B. schlosseri is a sequential (protogynous) hermaphrodite, and in a colony, eggs are ovulated about two days before the peak of sperm emission.[36] Thus self-fertilization is avoided, and cross-fertilization is favored. Although avoided, self-fertilization is still possible in B. schlosseri. Self-fertilized eggs develop with a substantially higher frequency of anomalies during cleavage than cross-fertilized eggs (23% vs. 1.6%).[36] Also a significantly lower percentage of larvae derived from self-fertilized eggs metamorphose, and the growth of the colonies derived from their metamorphosis is significantly lower. These findings suggest that self-fertilization gives rise to inbreeding depression associated with developmental deficits that are likely caused by expression of deleterious recessive mutations.[35]
A model tunicate
Oikopleura dioica (class Appendicularia) is a semelparous organism, reproducing only once in its lifetime. It employs an original reproductive strategy in which the entire female germ-line is contained within an ovary that is a single giant multinucleate cell termed the "coenocyst".[37] O. dioica can be maintained in laboratory culture, and is of growing interest as a model organism because of its phylogenetic position within the closest sister group to vertebrates.[12]
Invasive species
Over the past few decades, tunicates (notably of the genera Didemnum and Styela) have been invading coastal waters in many countries. The carpet tunicate (Didemnum vexillum) has taken over a 6.5 sq mi (17 km2) area of the seabed on the Georges Bank off the northeast coast of North America, covering stones, molluscs, and other stationary objects in a dense mat.[38] D. vexillum, Styela clava and Ciona savignyi have appeared and are thriving in Puget Sound and Hood Canal in the Pacific Northwest.[39]
Invasive tunicates usually arrive as fouling organisms on the hulls of ships, but may also be introduced as larvae in ballast water. Another possible means of introduction is on the shells of molluscs brought in for marine cultivation.[39] Current research indicates many tunicates previously thought to be indigenous to Europe and the Americas are, in fact, invaders. Some of these invasions may have occurred centuries or even millennia ago. In some areas, tunicates are proving to be a major threat to aquaculture operations.[40]
Use by humans
Medical uses
Tunicates contain a host of potentially useful chemical compounds, including:
- Didemnins, effective against various types of cancer as antivirals and immunosuppressants
- Aplidine, effective against various types of cancer
- Trabectedin, effective against various types of cancer
Tunicates can correct abnormalities over a series of generations, and a similar regenerative process may be possible for humans. The mechanisms underlying the phenomenon may lead to insights about the potential of cells and tissues to be reprogrammed and regenerate compromised human organs. [41]
As food

Various Ascidiacea species are consumed as food around the world. In Japan and Korea, the sea pineapple (Halocynthia roretzi) is the main species eaten. It is cultivated on dangling cords made of palm fronds. In 1994, over 42,000 tons were produced, but since then, mass mortality events have occurred among the farmed sea squirts (the tunics becoming soft), and only 4,500 tons were produced in 2004.[42]
Other uses
The use of tunicates as a source of biofuel is being researched. The cellulose body wall can be broken down and converted into ethanol, and other parts of the animal are protein-rich and can be converted into fish feed. Culturing tunicates on a large scale may be possible and the economics of doing so are attractive. As tunicates have few predators, their removal from the sea may not have profound ecological impacts. Being sea-based, their production does not compete with food production as does the cultivation of land-based crops for biofuel projects.[43]
Some tunicates are used as model organisms. Ciona intestinalis and Ciona savignyi have been used for developmental studies. Both species' mitochondrial[44][45] and nuclear[46][47] genomes have been sequenced. The nuclear genome of the appendicularian Oikopleura dioica appears to be one of the smallest among metazoans[48] and this species has been used to study gene regulation and the evolution and development of chordates.[49]
See also
- Vetulicolia – crown-group chordates which are probably the sister group of modern tunicates
- Donald I. Williamson – claimed hybridisation
References
- ↑ Fedonkin, M. A.; Vickers-Rich, P.; Swalla, B. J.; Trusler, P.; Hall, M. (2012). "A new metazoan from the Vendian of the White Sea, Russia, with possible affinities to the ascidians". Paleontological Journal 46: 1. doi:10.1134/S0031030112010042.
- ↑ 2.0 2.1 Sanamyan, Karen (2013). "Tunicata". World Register of Marine Species. Retrieved 2013-04-04.
- ↑ Nielsen, C. (2012). "The authorship of higher chordate taxa". Zoologica Scripta 41 (4): 435–436. doi:10.1111/j.1463-6409.2012.00536.x.
- ↑ 4.0 4.1 Tatián, Marcos; Lagger, Cristian; Demarchi, Milagros; Mattoni, Camilo (2011). "Molecular phylogeny endorses the relationship between carnivorous and filter-feeding tunicates (Tunicata, Ascidiacea)". Zoologica Scripta 40 (6): 603–612. doi:10.1111/j.1463-6409.2011.00493.x.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 5.9 5.10 5.11 5.12 5.13 5.14 5.15 5.16 Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology, 7th edition. Cengage Learning. pp. 940–956. ISBN 81-315-0104-3.
- ↑ Foster, M. (ed.); Sedgwick, Adam (ed.); The Works of Francis Maitland Balfour. Vol. III. Memorial edition. Pub: Macmillan and co. 1885. May be downloaded from
- ↑ Tunicata World Register of Marine Species. Retrieved 2011-11-12.
- ↑ Tunicata Lamarck, 1816 Integrated Taxonomic Information System. Retrieved 2011-11-12.
- ↑ "Sea squirts and sea tulips". Australian Museum. Retrieved 2013-09-25.
- ↑ "Sea squirt". Dictionary.com. Retrieved 2013-09-25.
- ↑ "Sea pork, Aplidium stellatum". Smithsonian at Fort Pierce. Retrieved 2013-09-25.
- ↑ 12.0 12.1 Delsuc, F.; Brinkmann, H.; Chourrout, D.; Philippe, H. (2006). "Tunicates and not cephalochordates are the closest living relatives of vertebrates". Nature 439 (7079): 965–968. doi:10.1038/nature04336. PMID 16495997.
- ↑ Delsuc, F.; Tsagkogeorga, G.; Lartillot, N.; Philippe, H. (2008). "Additional molecular support for the new chordate phylogeny". Genesis 46 (11): 592–604. doi:10.1002/dvg.20450. PMID 19003928.
- ↑ Singh, T. R.; Tsagkogeorga, G.; Delsuc, F.; Blanquart, S.; Shenkar, N.; Loya, Y.; Douzery, E. J.; Huchon, D. (2009). "Tunicate mitogenomics and phylogenetics: peculiarities of the Herdmania momus mitochondrial genome and support for the new chordate phylogeny". BMC Genomics 10: 534. doi:10.1186/1471-2164-10-534. PMC 2785839. PMID 19922605.
- ↑ Jefferies, R. P. S. (1991) in Biological Asymmetry and Handedness (eds Bock, G. R.; Marsh, J.) pp. 94-127 (Wiley, Chichester).
- ↑ Zeng, L.; Swalla, B. J. (2005). "Molecular phylogeny of the protochordates: chordate evolution". Can. J. Zool. 83: 24–33. doi:10.1139/z05-010.
- ↑ Tsagkogeorga, G.; Turon, X.; Hopcroft, R. R.; Tilak, M. K.; Feldstein, T.; Shenkar, N.; Loya, Y.; Huchon, D.; Douzery, E. J.; Delsuc, F. (2009). "An updated 18S rRNA phylogeny of tunicates based on mixture and secondary structure models". BMC Evolutionary Biology 9: 187. doi:10.1186/1471-2148-9-187. PMC 2739199. PMID 19656395.
- ↑ Chen, Jun-Yuan; Huang, Di-Ying; Peng, Qing-Qing; Chi, Hui-Mei; Wang,Xiu-Qiang; Feng, Man (2003). "The first tunicate from the Early Cambrian of South China". Proceedings of the National Academy of Sciences 100 (14): 8314–8318. doi:10.1073/pnas.1431177100. PMC 166226. PMID 12835415.
- ↑ Palmer, T. J.; Wilson, M. A. (1988). "Parasitism of Ordovician bryozoans and the origin of pseudoborings" (PDF). Palaeontology 31: 939–949.
- ↑ Vickers-Rich P. (2007). "Chapter 4. The Nama Fauna of Southern Africa". In: Fedonkin, M. A.; Gehling, J. G.; Grey, K.; Narbonne, G. M.; Vickers-Rich, P. "The Rise of Animals: Evolution and Diversification of the Kingdom Animalia", Johns Hopkins University Press. pp. 69-87
- ↑ 21.0 21.1 Fedonkin, M. A.; Vickers-Rich, P.; Swalla, B.; Trusler, P.; Hall, M. (2008). "A Neoproterozoic chordate with possible affinity to the ascidians: New fossil evidence from the Vendian of the White Sea, Russia and its evolutionary and ecological implications". HPF-07 Rise and fall of the Ediacaran (Vendian) biota. International Geological Congress - Oslo 2008.
- ↑ "Introduction to the Urochordata". University of California Museum of Paleontology. Retrieved 2013-04-07.
- ↑ Syvanen, M.; Ducore, J. (2010). "Whole genome comparisons reveals a possible chimeric origin for a major metazoan assemblage". Journal of Biological Systems 18 (2): 261–275. doi:10.1142/S0218339010003408.
- ↑ Lawton, G. (2009-01-21). "Uprooting Darwin's tree" (PDF). New Scientist. Retrieved 2013-04-19.
- ↑ 25.0 25.1 25.2 25.3 Dorit, R. L.; Walker, W. F.; Barnes, R. D. (1991). Zoology. Saunders College Publishing. pp. 802–804. ISBN 978-0-03-030504-7.
- ↑ Matthysse, Ann G.; Deschet, Karine; Williams, Melanie; Marry, Mazz; White, Alan R.; Smith, William C. (2004). "A functional cellulose synthase from ascidian epidermis". PNAS 101 (4): 986–991. doi:10.1073/pnas.0303623101. PMC 327129. PMID 14722352.
- ↑ Hirose, Euichi; Nakashima, Keisuke; Nishino, Atsuo (2011). "Is there intracellular cellulose in the appendicularian tail epidermis? A tale of the adult tail of an invertebrate chordate". Communicative & Integrative Biology 4 (6): 768–771. doi:10.4161/cib.17757.
- ↑ Hall, D. A.; Saxl, Hedwig (1961). "Studies of Human and Tunicate Cellulose and of their Relation to Reticulin". Proceedings of the Royal Society of London 155 (959): 202–217. doi:10.1098/rspb.1961.0066. JSTOR 90413.
- ↑ Sasakura, Yasunori; Nakashima, Keisuke; Awazu, Satoko; Matsuoka, Terumi; Nakayama, Akie; Azuma, Jun-ichi; Satoh, Nori (2005). "Transposon-mediated insertional mutagenesis revealed the functions of animal cellulose synthase in the ascidian Ciona intestinalis". Proceedings of the National Academy of Sciences 102 (42): 15134–15139. doi:10.1073/pnas.0503640102.
- ↑ 30.0 30.1 Cavanihac, Jean-Marie (2000). "Tunicates extraordinaire". Microscope UK. Retrieved 2011-12-07.
- ↑ Dennett, Daniel C. (1991). Consciousness Explained. Little Brown & Co. p. 177. ISBN 978-0316-18065-8.
- ↑ Parmentier, Jan (1998). "Botryllus: A colonial ascidian". Microscope UK. Retrieved 2013-04-07.
- ↑ Holland, Linda Z. (2007). "Developmental biology: A chordate with a difference". Nature 447 (1): 153–155. doi:10.1038/447153a. PMID 17495912.
- ↑ 34.0 34.1 Sawada H, Morita M, Iwano M (August 2014). "Self/non-self recognition mechanisms in sexual reproduction: new insight into the self-incompatibility system shared by flowering plants and hermaphroditic animals". Biochem. Biophys. Res. Commun. 450 (3): 1142–8. doi:10.1016/j.bbrc.2014.05.099. PMID 24878524.
- ↑ 35.0 35.1 Bernstein, H; Hopf, FA; Michod, RE (1987). "The molecular basis of the evolution of sex". Adv Genet 24: 323–70. PMID 3324702.
- ↑ 36.0 36.1 Gasparini, F; Manni, L; Cima, F; Zaniolo, G; Burighel, P; Caicci, F; Franchi, N; Schiavon, F; Rigon, F; Campagna, D; Ballarin, L (July 2014). "Sexual and asexual reproduction in the colonial ascidian Botryllus schlosseri". Genesis 53: 105–20. doi:10.1002/dvg.22802. PMID 25044771.
- ↑ Ganot P, Bouquet JM, Kallesøe T, Thompson EM (February 2007). "The Oikopleura coenocyst, a unique chordate germ cell permitting rapid, extensive modulation of oocyte production". Dev. Biol. 302 (2): 591–600. doi:10.1016/j.ydbio.2006.10.021. PMID 17126826.
- ↑ "Have You Seen This Tunicate?". NOAA Fisheries Service. 2004-11-19. Retrieved 2011-12-07.
- ↑ 39.0 39.1 Dornfeld, Ann (2008-05-01). "Invasive Tunicates of Washington State". NPR. Retrieved 2013-04-06.
- ↑ "Marine Nuisance Species". Woodshole Science Center. Retrieved 2011-12-07.
- ↑ "Sea Squirt, Heal Thyself: Scientists Make Major Breakthrough in Regenerative Medicine". Sciencedaily.com. 2007-04-24. Retrieved 2011-12-07.
- ↑ "Sea squirt". Korea-US Aquaculture. Retrieved 2013-04-06.
- ↑ "Biofuel made from marine filter feeders? Tunicates usable as source of biofuels". Cleantechnica. 2013-03-26. Retrieved 2013-04-06.
- ↑ Iannelli, F.; Pesole, G.,; Sordino, P.; Gissi, C. (2007). "Mitogenomics reveals two cryptic species in Ciona intestinalis". Trends Genet. 23 (9): 419–422. doi:10.1016/j.tig.2007.07.001. PMID 17640763.
- ↑ Yokobori, S.; Watanabe, Y.; Oshima, T. (2003). "Mitochondrial genome of Ciona savignyi (Urochordata, Ascidiacea, Enterogona): Comparison of gene arrangement and tRNA genes with Halocynthia roretzi mitochondrial genome". J. Mol. Evol. 57 (5): 574–587. doi:10.1007/s00239-003-2511-9. PMID 14738316.
- ↑ Dehal, P.; Satou, Y.; Campbell, R. K.; Chapman, J., Degnan, B., De Tomaso, A.; Davidson, B.; Di Gregorio, A.; Gelpke, M.; Goodstein, D. M.; Harafuji, N.; Hastings, K. E.; Ho, I.; Hotta, K.; Huang, W.; Kawashima, T.; Lemaire, P.; Martinez, D.; Meinertzhagen, I. A.; Necula, S.; Nonaka, M.; Putnam, N.; Rash, S.; Saiga, H.; Satake, M.; Terry, A.; Yamada L.; Wang, H. G.; Awazu, S.; Azumi, K.; Boore, J.; Branno, M.; Chin-Bow, S.; DeSantis, R.; Doyle, S., Francino, P.; Keys, D. N.; Haga, S.; Hayashi, H.; Hino, K.; Imai, K. S.; Inaba, K.; Kano, S.; Kobayashi, K.; Kobayashi, M.; Lee, B. I.; Makabe, K. W.; Manohar, C.; Matassi, G.; Medina, M.; Mochizuki, Y.; Mount, S.; Morishita, T.; Miura, S.; Nakayama, A.; Nishizaka, S.; Nomoto, H.; Ohta, F.; Oishi, K.; Rigoutsos, I.; Sano, M.; Sasaki, A.; Sasakura, Y.; Shoguchi, E.; Shin-i, T.; Spagnuolo, A.; Stainier, D.; Suzuki, M. M.; Tassy, O.; Takatori, N.; Tokuoka, M.; Yagi, K.; Yoshizaki, F.; Wada, S.; Zhang C.; Hyatt, P. D.; Larimer, F.; Detter, C.; Doggett, N.; Glavina, T.; Hawkins, T.; Richardson, P.; Lucas, S.; Kohara, Y.; Levine, M.; Satoh, N.; Rokhsar, D. S. (2002). "The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins". Science 298 (5601): 2157–2167. doi:10.1126/science.1080049. PMID 12481130.
- ↑ Small, K. S.; Brudno, M.; Hill, M. M.; Sidow, A. (2007). "A haplome alignment and reference sequence of the highly polymorphic Ciona savignyi genome". Genome Biol. 8 (3): R41. doi:10.1186/gb-2007-8-3-r41. PMC 1868934. PMID 17374142.
- ↑ Seo, H. C.; Kube, M.; Edvardsen, R. B.; Jensen, M. F.; Beck, A.; Spriet, E.; Gorsky, G.; Thompson. E. M.; Lehrach, H.; Reinhardt, R.; Chourrout, D. (2001). "Miniature genome in the marine chordate Oikopleura dioica". Science 294 (5551): 2506–2506. doi:10.1126/science.294.5551.2506. PMID 11752568.
- ↑ Clarke, T.; Bouquet, JM; Fu, X; Kallesøe, T.; Schmid, M; Thompson, E.M. (2007). "Rapidly evolving lamins in a chordate, Oikopleura dioica, with unusual nuclear architecture". Gene 396 (1): 159–169. doi:10.1016/j.gene.2007.03.006. PMID 17449201.
External links
| Wikimedia Commons has media related to Tunicata. |
| Wikispecies has information related to: Urochordata |
- The Tunicate Web Portal
- Dutch Ascidians: Extensive database of images from around the world
- Aniseed: A model organism database for ascidians including Ciona intestinalis and Halocynthia roretzi
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||