Tubocurarine chloride
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
| 6,6'-dimethoxy-2,2,2',2'-tetramethyltubocuraran-2,2'-diium-7',12'-diol | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a682860 |
| |
| |
| IV | |
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Protein binding | 50% |
| Half-life | 1-2 hours |
| Identifiers | |
|
57-95-4 6989-98-6 (chloride hydrochloride pentahydrate) | |
| M03AA02 | |
| PubChem | CID 6000 |
| DrugBank |
DB01199 |
| ChemSpider |
5778 |
| UNII |
900961Z8VR |
| ChEBI |
CHEBI:9774 |
| ChEMBL |
CHEMBL1687 |
| Chemical data | |
| Formula | C37H42Cl2N2O6 |
| 624.765 g/mol | |
|
SMILES
| |
| |
| | |
Tubocurarine (also known as d-tubocurarine or DTC) is a toxic alkaloid and skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in anesthesia to provide skeletal muscle relaxation during surgery or mechanical ventilation. Unlike a number of other related skeletal muscle relaxants, it is now rarely considered clinically to facilitate endotracheal intubation.
Tubocurarine is classified as a long-duration,[1] antagonist for Nicotinic acetylcholine receptor.[2] It is the active agent of one of the forms of curare.
Currently, tubocurarine is rarely used as an adjunct for clinical anesthesia because safer alternatives, such as cisatracurium and rocuronium, are available.
History
Tubocurare is a naturally occurring mono-quaternary alkaloid obtained from the bark of the South American plant Chondrodendron tomentosum, a climbing vine known to the European world since the Spanish conquest of South America. Curare had been used as a source of arrow poison by South American natives to hunt animals, and they were able to eat the animals' contaminated flesh subsequently without any untoward effects, because tubocurarine cannot easily cross mucous membranes. Tubocurarine is thus effective only if given parenterally, as demonstrated by Bernard, who also showed the site of its action was at the neuromuscular junction.[3] Virchow and Munter confirmed the paralyzing action was limited to voluntary and not involuntary muscles.[4] Thus, a conscious individual administered this agent will be unable to move any voluntary muscles, including the diaphragm: a large enough dose will therefore result in death from respiratory failure unless artificial ventilation is initiated.
The word "curare" comes from the South American Indian name for the arrow poison, ourare. Presumably, the initial syllable was pronounced with a heavy glottal stroke. Tubocurarine is so-called because the plant samples containing the curare were stored and shipped to Europe in tubes. Likewise, curare stored in calabash containers was called calabash curare.
Structurally, tubocurarine is a benzylisoquinoline derivative. For many years, its structure, when first elucidated in 1948,[5] was wrongly thought to be bis-quaternary: in other words, it was thought to be an N,N-dimethylated alkaloid. In 1970, the correct structure was finally established,[6] showing one of the two nitrogens to be tertiary, actually a mono-N-methylated alkaloid.
Griffith and Johnson are credited with pioneering the formal clinical introduction of tubocurarine as an adjunct to anesthetic practice on 23 January 1942, at the Montreal Homeopathic Hospital.[7] In this sense, tubocurarine is the prototypical adjunctive neuromuscular blocking agent. However, others before Griffith and Johnson had attempted use of tubocurare in several situations:[8][9][10] some under controlled study conditions[11][12] while others not quite controlled and remained unpublished.[13] Regardless, all in all some ~30,000 patients had been given tubocurare by 1941, although it was Griffith and Johnson's 1942 publication[7] that provided the impetus to the standard use of neuromuscular blocking agents in clinical anesthestic practice - a revolution that rapidly metamorphosized into the standard practice of "balanced" anesthesia: the triad of barbiturate hypnosis, light inhalational anesthesia and muscle relaxation.[14] The technique as described by Gray and Halton was widely known as the "Liverpool technique",[14] and became the standard anesthetic technique in England in the 1950s and 1960s for patients of all ages and physical status. Present clinical anesthetic practice still employs the central principle of balanced anesthesia though with some differences to accommodate subsequent technological advances and introductions of new and better gaseous anesthetic, hypnotic and neuromuscular blocking agents, and tracheal intubation, as well as monitoring techniques that were nonexistent in the day of Gray and Halton: pulse oximetry, capnography, peripheral nerve stimulation, noninvasive blood pressure monitoring, etc.
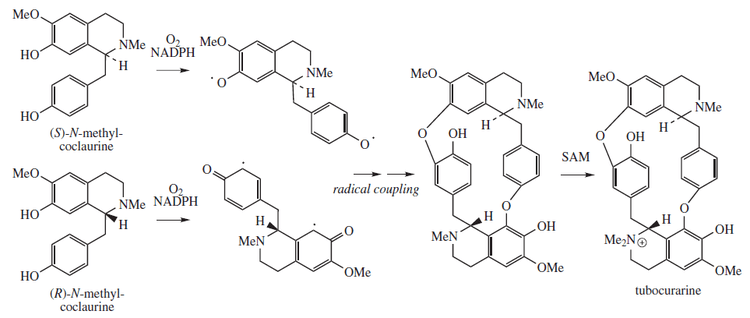
Biosynthesis
Tubocurarine biosynthesis involves a radical coupling of two enantiomeric tetrahydrobenzylisoquinolines, more specifically, the two enantiomers of N-methyl-coclaurine. (R) and (S)-N-methyl-coclaurine come from a Mannich-like reaction between dopamine and 4-hydroxyphenyl-acetaldehyde, facilitated by norcoclaurine synthase (NCS). Both dopamine and 4-hydroxyphenylacetyladehyde originate from L-tyrosine. The biosynthetic pathway is described in more detail in the figures. Methylation of the amine and hydroxyl substituents are facilitated by S-adenosyl methionine (SAM).[15]
Clinical pharmacology and pharmacokinetics
Tubocurarine has a time of onset of 300 seconds or more (which is relatively slow among neuromuscular-blocking drugs), and has a duration of action of 60 to 120 minutes (which is relatively long time).[16]
It also causes histamine release,[17] now a recognized hallmark of the tetrahydroisioquinolinium class of neuromuscular blocking agents. The amount of histamine release in some instances following tubocurarine administration is such that it is contraindicated in asthmatics and patients with allergies. However, the main disadvantage in the use of tubocurarine is its significant ganglion-blocking effect,[18] that manifests as hypotension,[19] in many patients; this constitutes a relative contraindication to its use in patients with myocardial ischaemia.
Because of the shortcomings of tubocurare, much research effort was undertaken soon after its clinical introduction to find a suitable replacement. The efforts unleashed a multitude of compounds borne from structure-activity relations developed from the tubocurare molecule. Some key compounds that have seen clinical use are identified in the muscle relaxants template box below. Of the many tried as replacements, only a few enjoyed as much popularity as tubocurarine: pancuronium, vecuronium, rocuronium, atracurium, and cisatracurium. Succinylcholine is a widely used paralytic drug which acts by activating, instead of blocking, the ACh receptor.
The potassium channel blocker tetraethylammonium (TEA) has been shown to reverse the effects of tubocurarine. It is thought to do so by increasing ACh release, which counteracts the antagonistic effects of tubocurarine on the ACh receptor.
References
- ↑ Thompson MA (1980). "Muscle relaxant drugs". Br J Hosp Med 23 (2): 153–4, 163–4, 167–8 passim. PMID 6102875.
- ↑ Wenningmann I, Dilger JP (October 2001). "The kinetics of inhibition of nicotinic acetylcholine receptors by (+)-tubocurarine and pancuronium". Molecular Pharmacology 60 (4): 790–6. PMID 11562442.
- ↑ Bernard C (1856). "Analyse physiologie des propriétés des actions de curare et de la nicotine sure systèmes musculaire et nerveux au moyen du curare". Compt. Rend. 43: 305–319.
- ↑ Bechter AM (1977). "The civilizing of curare: a history of its development and introduction into anesthesiology". Anesth Analg 56 (2): 305–319. doi:10.1213/00000539-197703000-00032. PMID 322548.
- ↑ King H (1948). "64. Curare alkaloids. Part VII. Constitution of dextrotubocurarine chloride". J Chem Soc: 265. doi:10.1039/jr9480000265.
- ↑ Everett AJ, Lowe LA, Wilkinson S (1970). J Chem Soc, Chem Commun: 1020. Missing or empty
|title=(help) - ↑ 7.0 7.1 Griffith HR, Johnson GE (1942). "The use of curare in general anesthesia". Anesthesiol 3 (4): 418–420. doi:10.1097/00000542-194207000-00006.
- ↑ Läwen A (1912). "Ueber die verbindung der lokakanaesthesie und epidurale injektion anesthesiernder losungen bei tabischen magenkrisen". Beitr Klin Chir 80: 168–189.
- ↑ Wilkinson DJ (1991). "Dr. F.P. de Caux - the first user of curare for anaesthesia in England". Anaesthesia 46 (1): 49–51. doi:10.1111/j.1365-2044.1991.tb09317.x. PMID 1996757.
- ↑ Bennett AE (1941). "Curare: a preventive of traumatic complications in electroconvulsive shock therapy". Am J Psychiatry 97: 1040–1060.
- ↑ West R (1984). "An excursion into pharmacology: curare in medicine". Medical History 28 (4): 391–405. doi:10.1017/s0025727300036279. PMC 1140012. PMID 6390032.
- ↑ Burman MS (1939). "Therapeutic use of curare and erythroidine hydrochloride for spastic and dystonic states". Arch Neurol Psychiat 41: 307–327. doi:10.1001/archneurpsyc.1939.02270140093008.
- ↑ Bevan DR. (1992) "Curare". In: Maltby JR, Shephard DAE (Eds.), Harold Griffith - His Life and Legacy; Suppl. to Can J Anaesth Vol. 39 (1); 49-55.
- ↑ 14.0 14.1 Gray TC, Halton J (1946). "Technique for the use of d-tubocurarine chloride with balanced anaesthesia". Br Med J 2: 293–295. PMID 20996123.
- ↑ Dewick, P. M. Medicinal Natural Products; a Biosynthetic Approach. 3rd ed.; John Wiley and Sons Ltd.: 2009.
- ↑ Page 151 in: Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4. OCLC 51622037.
- ↑ Maclagen J. (1976) In: Zaimis E. (Ed.), "Neuromuscular Junction". Hand. Exp. Pharm.; Vol. 42; Springer-Verlag, Berlin: 421-486.
- ↑ Bowman WC, Webb SN (1972). "Neuromuscular blocking and ganglion blocking activities of some acetylcholine antagonists in the cat". J Pharm Pharmacol 24 (10): 762–72. doi:10.1111/j.2042-7158.1972.tb08880.x. PMID 4403972.
- ↑ Coleman AJ, Downing JW, Leary WP, Moyes DG (1972). "The immediate cardiovascular effects of pancuronium, alcuronium and tubocurarine in man". Anaesthesia 27 (4): 415–22. doi:10.1111/j.1365-2044.1972.tb08247.x. PMID 4264060.
External links
- Curare - History, Tubocurarine, a more detailed historical account of tubocurare
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||