Triphenylphosphine dichloride
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dichlorotriphenyl-λ5-phosphane | |||
| Other names
Dichlorotriphenylphosphorane | |||
| Identifiers | |||
| 2526-64-9 | |||
| Properties | |||
| C18H15Cl2P | |||
| Molar mass | 333.19 g/mol | ||
| Melting point | 176 °C (349 °F; 449 K)[1] 85-100 °C[2] | ||
| Related compounds | |||
| Related compounds |
Phosphoranes Triphenylphosphine Phosphorus trichloride Phosphorus pentachloride Phosphorus halides Tetraphenylphosphonium chloride | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Triphenylphosphine dichloride, Ph3PCl2, is a chlorinating agent widely used in organic chemistry. Applications include the conversion of alcohols and ethers to alkyl chlorides, the cleavage of epoxides to vicinal dichlorides and the chlorination of carboxylic acids to acyl chlorides.[2]
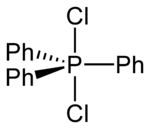
Structure
In polar solvents such as acetonitrile or dichloromethane solutions, Ph3PCl2 adopts an ionic phosphonium salt structure, [Ph3PCl+]Cl−,[3] whereas in non-polar solvents like diethyl ether it exists as a non-solvated trigonal bipyramidal molecule.[4] Two [Ph3PCl+] species can also adopt an unusual dinuclear ionic structure—both interacting with a Cl− via long Cl–Cl contacts.[3]
Synthesis
Triphenylphosphine dichloride is usually prepared fresh by the addition of chlorine to triphenylphosphine.
- Ph3P + Cl2 → Ph3PCl2
Both reagents are typically used in solution to ensure the correct stoichiometry.[2]
Alternatively, Ph3PCl2 can be obtained by chlorination of triphenylphosphine oxide with, for example, phosphorus trichloride, as in Grignard's original 1931 synthesis.[1]
References
- ↑ 1.0 1.1 V. Grignard, J. Savard (1931). Compt. Rend. 192: 592–5. Missing or empty
|title=(help) - ↑ 2.0 2.1 2.2 e-EROS Encyclopedia of Reagents for Organic Synthesis, doi:10.1002/047084289X.rt371
- ↑ 3.0 3.1 S. M. Godfrey, C. A. McAuliffe, R. G. Pritchard, J. M. Sheffield (1996). "An X-ray crystallorgraphic study of the reagent Ph3PCl2; not charge-transfer, R3P–Cl–Cl, trigonal bipyramidal or [R3PCl]Cl but an unusual dinuclear ionic species, [Ph3PCl+⋯Cl–⋯+CIPPH3]Cl containing long Cl–Cl contacts". Chem. Commun. (22): 2521–2522. doi:10.1039/CC9960002521.
- ↑ S. M. Godfrey, C. A. McAuliffe, J. M. Sheffield (1998). "Structural dependence of the reagent Ph3PCl2 on the nature of the solvent, both in the solid state and in solution; X-ray crystal structure of trigonal bipyramidal Ph3PCl2, the first structurally characterised five-coordinate R3PCl2 compound". Chem. Commun. (8): 921–922. doi:10.1039/a800820e.




