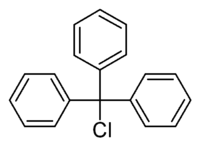
Triphenylmethyl chloride
 | |
| Names | |
|---|---|
| IUPAC name
[chloro-di(phenyl)methyl]benzene | |
| Other names
1,1',1"-(chloromethanetriyl)tribenzene | |
| Identifiers | |
| 76-83-5 | |
| ChemSpider | 17344583 |
| |
| Jmol-3D images | Image |
| PubChem | 6456 |
| |
| Properties | |
| C19H15Cl | |
| Molar mass | 278.7754 g/mol |
| Appearance | white to yellow solid |
| Density | 1.141g/cm3 |
| Melting point | 109 to 112 °C (228 to 234 °F; 382 to 385 K) |
| Boiling point | 230 °C (446 °F; 503 K) (at 20 mmHg) and 374.3 °C (at 760 mmHg) |
| Solubility | soluble in chloroform, benzene, acetone,[1] ether, THF, hexane[2] |
| Hazards | |
| MSDS | Corvine Chemicals MSDS |
| Flash point | 177.9 °C (352.2 °F; 451.0 K) |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Triphenylmethyl chloride or trityl chloride (TrCl) is a white solid with the chemical formula C19H15Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group.
Preparation
Triphenylmethyl chloride is commercially available. It may be prepared by the reaction of triphenylmethanol with acetyl chloride, or by the Friedel-Crafts alkylation of benzene with carbon tetrachloride to give the trityl chloride-aluminium chloride adduct, which is then hydrolyzed.[3]
Reactions
Triphenylmethylsodium can be prepared from trityl chloride dissolved in an aprotic solvent and sodium:[4]
- (C6H5)3CCl + 2 Na → (C6H5)3CNa + NaCl
Reaction with silver hexafluorophosphate gives triphenylmethyl hexafluorophosphate.
See also
References
- ↑ http://www.sciencelab.com/msds.php?msdsId=9925340
- ↑ http://www.scbt.com/datasheet-258321-trityl-chloride.html
- ↑ W. E. Bachmann; C. R. Hauser; Boyd E. Hudson, Jr. (1955). "Triphenylchloromethane". Org. Synth.; Coll. Vol. 3, p. 841
- ↑ W. B. Renfrow Jr and C. R. Hauser (1943). "Triphenylmethylsodium". Org. Synth.; Coll. Vol. 2, p. 607