Trimethylsilanol
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trimethylsilanol | |||
| Systematic IUPAC name
Trimethylsilanol | |||
| Other names
Trimethylhydroxysilane | |||
| Identifiers | |||
| 1066-40-6 | |||
| ChemSpider | 59498 | ||
| EC number | 213-914-1 | ||
| |||
| Jmol-3D images | Image | ||
| MeSH | Trimethylsilanol | ||
| PubChem | 66110 | ||
| |||
| Properties | |||
| Molecular formula |
C3H10OSi | ||
| Molar mass | 90.20 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Boiling point | 99 °C (210 °F; 372 K) | ||
| Vapor pressure | 21 mbar (20 °C) [1] | ||
| Related compounds | |||
| Related compounds |
tert-Butanol | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| Infobox references | |||
Trimethylsilanol (TMS) is an organosilicon compound with the formula (CH3)3SiOH. The Si centre bears three methyl groups and one hydroxyl group. It is a colourless volatile liquid.[2]
TMS is used to apply hydrophobic coating on silicate surfaces. It reacts with the silanol groups of the substrate, coating the surface with a layer of hydrophobic methyl groups. A commercial example is Magic Sand.
Occurrence
TMS is a common contaminant in the atmospheres of spacecraft, where it arises from the degradation of silicone-based materials.[3] Specifically, it is the volatile product from the hydrolysis of polydimethylsiloxane, which are generally terminated with trimethylsilyl groups:
- (CH3)3SiO[Si(CH3)2O]nR + H2O → (CH3)3SiOH + HO[Si(CH3)2O]nR
TMS and related volatile siloxanes are formed by hydrolysis of silicones-based containing materials, which are found in detergents and cosmetic products.
Traces of TMS, together with other volatile siloxanes, are present in biogas and landfill gas, again resulting from the degradation of silicones. As their combustion forms particles of silicates and microcrystalline quartz, which cause abrasion of combustion engine parts, they pose problems for the use of such gases in combustion engines.[4]
References
- ↑ Grubb, W.T.; Osthoff, R.C.: Physical Properties of Organosilicon Compounds. II. Trimethylsilanol and Triethylsilanol in J. Am. Chem. Soc. 75 (1953) 2230–2232; doi:10.1021/ja01105a061.
- ↑ Paul D. Lickiss "The Synthesis and Structure of Organosilanols" Advances in Inorganic Chemistry 1995, Volume 42, Pages 147–262 doi:10.1016/S0898-8838(08)60053-7
- ↑ Trimethylsilanol, Harold L. Kaplan, Martin E. Coleman, and John T. James Spacecraft Maximum Allowable Concentrations for Selected Airborne Contaminants, Volume 1 (1994)
- ↑ http://epics.ecn.purdue.edu/bgi/Documents/Fall%25202009/Removal_of_Siloxanes_.pdf
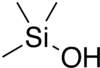
.png)