Trifluoromethanesulfonyl azide
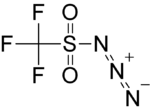 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
N-diazo-1,1,1-trifluoro-methanesulfonamide | |||
| Identifiers | |||
| 3855-45-6 | |||
| ChemSpider | 9161983 | ||
| |||
| Jmol-3D images | Image | ||
| PubChem | 10986786 | ||
| |||
| Properties | |||
| Molecular formula |
CF3N3O2S | ||
| Molar mass | 175.09 g·mol−1 | ||
| insoluble[1] | |||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Trifluoromethanesulfonyl azide or triflyl azide is an organic azide used as a reagent in organic synthesis.
Preparation
Trifluoromethanesulfonyl azide is not commercially available. It is prepared before use by reacting trifluoromethanesulfonic anhydride with sodium azide, traditionally in dichloromethane.[1] However, use of dichloromethane should be avoided because sodium azide is known to generate highly explosive azido-chloromethane and diazidomethane in situ by nucleophilic substitution on dichloromethane.[2] Moreover, the volatility of dichloromethane is a liability, as unsolvated triflyl azide is a detonation hazard.[2] The reaction may also be carried out in toluene,[3] acetonitrile, or pyridine.[4]
- Tf2O + NaN3 → TfN3 + NaOTf (Tf = CF3SO2)
The trifluoromethanesulfonic anhydride starting material is rather expensive, and the product is explosive, and does not store well. As a result, imidazole-1-sulfonyl azide has been developed as an alternative.[5]
Reactions
Trifluoromethanesulfonyl azide generally converts amines to azides. Trifluoromethanesulfonyl azide may be formed in situ from trifluoromethanesulfonic anhydride and sodium azide; it reacts with the amine present in a one-pot reaction.[1]
See also
References
- ↑ 1.0 1.1 1.2 C. J. Cavender and V. J. Shiner (1972). "Trifluoromethanesulfonyl azide. Its reaction with alkyl amines to form alkyl azides". The Journal of Organic Chemistry 37 (22): 3567–3569. doi:10.1021/jo00795a052.
- ↑ 2.0 2.1 Cavender, C. J.; Shiner, V. J., Jr. J. Org. Chem. (1972), 37, 3567.
- ↑ Titz, A.; Radic, Z.; Schwardt, O.; Ernst, B. Tetrahedron Lett. (2006), 47, 2383.
- ↑ R.-B. Yan, F. Yang, Y. Wu, L.-H. Zhang and X.-S. Ye (2005). "An efficient and improved procedure for preparation of triflyl azide and application in catalytic diazotransfer reaction". Tetrahedron Letters 46 (52): 8993–8995. doi:10.1016/j.tetlet.2005.10.103.
- ↑ E. D. Goddard-Borger and R. V. Stick (2007). "An Efficient, Inexpensive, and Shelf-Stable Diazotransfer Reagent: Imidazole-1-sulfonyl Azide Hydrochloride". Organic Letters 9 (19): 3797–3800. doi:10.1021/ol701581g. PMID 17713918.