Tridymite
| Tridymite | |
|---|---|
|
tabular tridymite crystals from Ochtendung, Eifel, Germany | |
| General | |
| Category | Oxide mineral |
| Formula (repeating unit) | SiO2 |
| Identification | |
| Formula mass | 60.08 |
| Color | Colorless, white |
| Crystal habit | Platy – sheet forms |
| Crystal system | several coexisting phases |
| Cleavage | {0001} indistinct, {1010} imperfect |
| Fracture | Brittle – conchoidal |
| Mohs scale hardness | 7 |
| Luster | Vitreous |
| Streak | white |
| Specific gravity | 2.25–2.28 |
| Optical properties | Biaxial (+), 2V=40–86° |
| Refractive index | nα=1.468–1.482 nβ=1.470–1.484 nγ=1.474–1.486 |
| Birefringence | δ < 0.004 |
| Pleochroism | Colorless |
| Other characteristics | non-radioactive, non-magnetic; fluorescent, short UV=dark red |
| References | [1] |
Tridymite is a high-temperature polymorph of silica and usually occurs as minute tabular white or colorless pseudo-hexagonal crystals, or scales, in cavities in felsic volcanic rocks. Its chemical formula is SiO2. Tridymite was first described in 1868 and the type location is in Hidalgo, Mexico. The name is from the Greek tridymos for triplet as tridymite commonly occurs as twinned crystal trillings.[1]
Structure

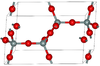
Tridymite can occur in seven crystalline forms. Two of the most common at standard pressure are known as α and β. The α-tridymite phase is favored at elevated temperatures (>870 °C) and it converts to β-cristobalite at 1470 °C.[2][3] However, tridymite does usually not form from pure β-quartz, one needs to add trace amounts of certain compounds to achieve this.[4] Otherwise the βquartz-tridymite transition is skipped and β-quartz transitions directly to cristobalite at 1050°C without occurrence of the tridymite phase.
| Name | Symmetry | Space group | T (°C) |
|---|---|---|---|
| HP (β) | Hexagonal | P63/mmc | 460 |
| LHP | Hexagonal | P6322 | 400 |
| OC (α) | Orthorhombic | C2221 | 220 |
| OS | Orthorhombic | 100–200 | |
| OP | Orthorhombic | P212121 | 155 |
| MC | Monoclinic | Cc | 22 |
| MX | Monoclinic | C1 | 22 |
In the table, M, O, H, C, P, L and S stand for monoclinic, orthorhombic, hexagonal, centered, primitive, low (temperature) and superlattice. T indicates the temperature, at which the corresponding phase is relatively stable, though the interconversions between phases are complex and sample dependent, and all these forms can coexist at ambient conditions.[3] Mineralogy handbooks often arbitrarily assign tridymite to the triclinic crystal system, yet use hexagonal Miller indices because of the hexagonal crystal shape (see infobox image).[1]
See also
| Wikisource has original text related to this article: |
References
- ↑ 1.0 1.1 1.2 Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W. and Nichols, Monte C. (ed.). "Tridymite". Handbook of Mineralogy (PDF). III (Halides, Hydroxides, Oxides). Chantilly, VA, US: Mineralogical Society of America. ISBN 0-9622097-2-4. Retrieved December 5, 2011.
- ↑ Kuniaki Kihara, Matsumoto T., Imamura M. (1986). "Structural change of orthorhombic-I tridymite with temperature: A study based on second-order thermal-vibrational parameters". Zeitschrift fur Kristallographie 177: 27–38. Bibcode:1986ZK....177...27K. doi:10.1524/zkri.1986.177.1-2.27.
- ↑ 3.0 3.1 3.2 William Alexander Deer; R. A. Howie; W. S. Wise (2004). Rock-Forming Minerals: Framework Silicates: Slica Minerals, Feldspathoids and the Zeolites. Geological Society. pp. 22–. ISBN 978-1-86239-144-4. Retrieved 2 January 2012.
- ↑ Heaney, P. J. (1994). "Structure and chemistry of the low-pressure silica polymorphs". Reviews in Mineralogy 29.
External links
| Wikimedia Commons has media related to Tridymite. |
| ||||||||||||||||||||||||||||||||
