Transition metal carbyne complex
Transition metal carbyne complexes are organometallic compounds with triple bond between carbon and the transition metal. This triple bond consists of a sigma bond and two pi bonds.[1] The HOMO of the carbyne ligand interacts with the LUMO of the metal to create the sigma bond. The two pi bonds are formed when the two HOMO orbitals of the metal back donate to the LUMO of the carbyne. They are also called metal alkylidynes—the carbon is a carbyne ligand. Such compounds are useful in organic synthesis of alkynes and nitriles. They have been the focus on much fundamental research.[2]
Synthesis
Transition metal carbyne complexes are most common for the early transition metals, especially Nb, Ta, Mo, W, and Re. They can also have low-valence metals as well as high-valence metals.
The first example of a metal carbyne complex was prepared by the Fischer school by treatment of Cr(CO)5(C(OMe)Ph) with boron trichloride:
- Cr(CO)5(C(OMe)Ph) + BCl3 → ClCr(CO)4(CPh) + CO + BCl2(OMe)
Many high valent carbyne complexes have since been prepared, often by dehydrohalogenation of carbene complexes. Alternatively, amino-substituted carbyne ligands sometime form upon protonation of electron-rich isonitrile complexes. Similarly, O-protonation of μ3-CO ligands in clusters gives hydroxycarbyne complex. Vinyl ligands have been shown to rearrange into carbyne ligands. Addition of electrophile to vinylidene ligands also affords carbyne complexes.[2]

Bridging alkylidyne ligands in cluster compounds
Some metal carbynes dimerize to give dimetallacyclobutadienes. In these complexes, the carbyne ligand serve as a bridging ligand.
Many cluster-bound carbyne complexes are known, typically with CO ligands. These compounds do not feature MC triple bonds, instead the carbyne carbon is tetrahedral. One of the best known are the tricobalt derivatives, which are prepared by treating cobalt carbonyl with haloforms:[3]
- 2 HCBr3 + 9/2 Co2(CO)8 → 2 HCCo3(CO)9 + 18 CO + 3 CoBr2
Structure
Monomeric metal carbyne complexes exhibit fairly linear M-C-R linkages according to X-ray crystallography, . The M-C distances is typically shorter than the M-C bond found in metal carbenes. The bond angle is generally greater than 170 degrees but it isn't exactly 180 degrees.[4] Analogous to Fischer vs Schrock carbenes; Fischer and Schrock carbynes are also known. Fischer carbynes usually have lower-valence metals and the ligands are pi-accepting ligands. Schrock carbynes on the other hand typically have high-valence metals and electron-donating ligands. In a Fischer carbyne the C-carbyne exhibits electrophilic behavior while Schrock carbynes display nucleophilic reactivity[5] Carbyne complexes have also been characterized by many methods including infrared Spectroscopy, Raman spectroscopy.[6] This these techniques you can determine a lot of useful information like bond lengths, bond angles and structures.

The first Fischer carbyne was isolated in 1973.[7] and the first Schrock carbyne was reported in 1978, 5 years later.[8]
Metal carbyne complexes also exhibit a large trans effect. The ligand opposite the carbyne is typically labile.
Reactions and applications
Metal alkylidyne complexes have mainly been used for specialized reactions in the laboratory, the main used being alkyne metathesis. Triply bridging carbynes are sometimes prepared by the condensation of terminal carbyne complexes with other metals. Transition metal carbyne complexes usually react with Lewis Acids at the C-carbyne. This reaction generally causes them to become transition metal carbene complexes. Depending on the charge of the carbyne complex depends on how well the complex will react with a nucleophile. A cationic carbyne complex will react with a nucleophile right at the C-carbyne. While a nucleophile will not react with the C-carbyne of a transition metal carbyne complex but instead it would react with the metal. This is due to the LUMO of the complexes caused by the electron orbitals of the metal and C-carbyne. Also the higher the energy of the d-orbitals belonging to an electron rich metal center the higher in energy of the metal carbon pi-bonds.[9] This improves the conditions for coupling.

Transition metal carbyne complexes can also react with electrophiles. The electrophile reacts with the C-carbyne to form a transition metal carbene structure. An example of this is shown below:

These complexes can also undergo photochemical reactions. This means that the transition metal carbyne complexes react with light. One cample of a photochemical reaction of a carbyne complex is the formation of a cyclopropenyl complex by an addition of an alkyne. This is also known as photooxidation. Generally when a transition metal carbyne complex undergoes photooxidation a new organic ring structure is formed. An example of this is shown below:
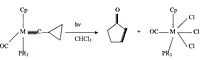
There are other photochemical reactions with carbyne complexes as well. Some of these include coupling of the carbyne ligand to a carbonyl, protonation of the carbyne carbon and conversion of the carbyne ligand into a pi-allyl.[10]
References
- ↑ Transition Metal Complexes with Terminal Carbyne Ligands Advances in Organometallic Chemistry Vol. 27. Page 52.
- ↑ 2.0 2.1 Elschenbroich, C. ”Organometallics” (2006) Wiley-VCH: Weinheim. ISBN 978-3-527-29390-2.
- ↑ Dietmar Seyferth, Mara O. Nestle, John S. Hallgren "μ3-Alkylidyne-Tris(Trigarbonylcobalt) Compounds: Organocobalt Cluster Complexes" Inorganic Syntheses, 1980, Volume 20, 224–226. doi:10.1002/9780470132517.ch52.
- ↑ Organometallic Chemistry 2nd Edition. Spessard, Gary O. Miessler, Gary L. Pages 439-449.
- ↑ W. A. Nugent, J. M. Mayer, Metal-Ligand Multiple Bonds, Wiley, New York, 1988.
- ↑ Transition Metal Carbyne Complexes; Kreißl, F.R
- ↑ E. O. Fischer, G. Kreis, C. G. Kreiter, J. Muller, G. Huttner, H. Lorenz. Angew. Chem. 1973, 85. 618.
- ↑ S. J. McLain, C. D. Wood, L. W. Messerle, R. R. Schrock, F. J. Hollander, W. J. Youngs, M. R. Churchill, J. Am. Chem. Soc. 1978, 100, 5962
- ↑ A. Mayr, C.M. Bastos, Prog. Inorg. Chem. 40,1 1992
- ↑ K.B. Kingsbury, J.D. Carter, L. McElwee-White, J. Chem. Soc., Chem. Commun. 1990, 624-625
| ||||||||||||||||||||||