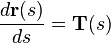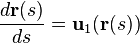Tractography

In neuroscience, tractography is a 3D modeling technique used to visually represent neural tracts using data collected by diffusion tensor imaging (DTI).[1] It uses special techniques of magnetic resonance imaging (MRI), and computer-based image analysis. The results are presented in two- and three-dimensional images.
In addition to the long tracts that connect the brain to the rest of the body, there are complicated neural networks formed by short connections among different cortical and subcortical regions. The existence of these bundles has been revealed by histochemistry and biological techniques on post-mortem specimens. Brain tracts are not identifiable by direct exam, CT, or MRI scans. This difficulty explains the paucity of their description in neuroanatomy atlases and the poor understanding of their functions.
The MRI sequences used look at the symmetry of brain water diffusion. Bundles of fiber tracts make the water diffuse asymmetrically in a tensor, the major axis parallel to the direction of the fibers. The asymmetry here is called anisotropy. There is a direct relationship between the number of fibers and the degree of anisotropy.
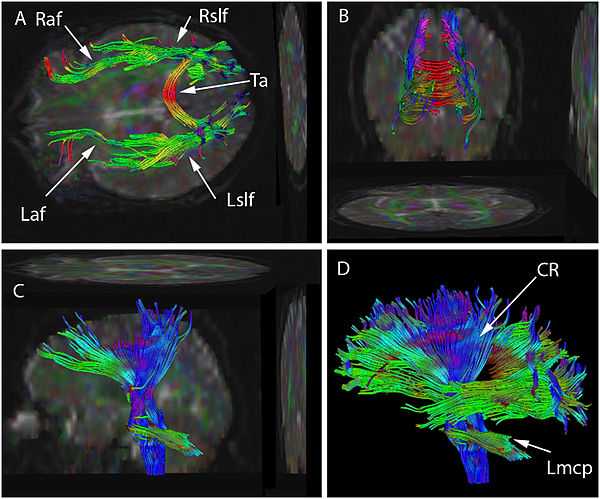
Figure legend: Diffusion tensor imaging (DTI) data have been used to seed various tractographic assessments of this patient's brain. These are seen in superior (A), posterior (B), and lateral views (C&D). The seeds have been used to develop arcuate and superior longitudinal fasciculi in (A) and (B), for brainstem, and corona radiata in (C), and as combined data sets in (D). Some of the two dimensional projections of the tractographic result are also shown. The data set may be rotated continuously into various planes to better appreciate the structure. Color has been assigned based on the dominant direction of the fibers. There is asymmetry in the tractographic fiber volume between the right and left arcuate fasciculus (Raf & Laf) (smaller on the left) and between the right and left superior longitudinal fasciculus (Rslf & Lslf) (smaller on the right). Also seen are Tapetum (Ta), Left corona radiata (Lcr) and Left middle cerebellar peduncle (Lmcp).
Mathematics
Using diffusion tensor MRI, one can measure the apparent diffusion coefficient at each voxel in the image, and after multilinear regression across multiple images, the whole diffusion tensor can be reconstructed.[2]
Suppose there is a fiber tract of interest in the sample. Following the Frenet–Serret formulas, we can formulate the space-path of the fiber tract as a parametrized curve:
where 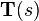 is the tangent vector of the curve. The reconstructed diffusion tensor
is the tangent vector of the curve. The reconstructed diffusion tensor 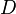 can be treated as a matrix, and we can easily compute its eigenvalues
can be treated as a matrix, and we can easily compute its eigenvalues 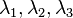 and eigenvectors
and eigenvectors 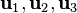 . By equating the eigenvector corresponding to the largest eigenvalue with the direction of the curve:
. By equating the eigenvector corresponding to the largest eigenvalue with the direction of the curve:
we can solve for 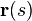 given the data for
given the data for 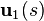 . This can be done using numerical integration, e.g., using Runge-Kutta, and by interpolating the principal eigenvectors.
. This can be done using numerical integration, e.g., using Runge-Kutta, and by interpolating the principal eigenvectors.
MRI technique

Tractography is performed using data from diffusion tensor imaging. Diffusion MRI, a method to produce images of the molecular diffusion process in tissues, was introduced in 1985, notably for its potential for neuroimaging.[3][4] An improvement of diffusion MRI, called Diffusion Tensor MRI or DTI [5][6] allows to fully characterize molecular diffusion in the 3 dimensions of space. Free diffusion occurs equally in all directions. This is termed "isotropic" diffusion. If the water diffuses in a medium with barriers, the diffusion will be uneven, which is termed "anisotropic" diffusion. In such a case, the relative mobility of the molecules from the origin has a shape different from a sphere. This shape is often modeled as an ellipsoid, and the technique is then called diffusion tensor imaging. Barriers can be many things—cell membranes, axons, myelin, etc.; but in white matter the principal barrier is the myelin sheath of axons. Bundles of axons provide a barrier to perpendicular diffusion and a path for parallel diffusion along the orientation of the fibers.
Anisotropic diffusion is expected to be increased in areas of high mature axonal order. Conditions where the myelin or the structure of the axon are disrupted, such as trauma, tumors, and inflammation reduce anisotropy, as the barriers are affected by destruction or disorganization.
Anisotropy is measured in several ways. One way is by a ratio called "fractional anisotropy" (FA). An anisotropy of "0" corresponds to a perfect sphere, whereas 1 is an ideal linear diffusion. Well-defined tracts have FA larger than 0.20. Few regions have FA larger than 0.90. The number gives information of how aspherical the diffusion is but says nothing of the direction.
Each anisotropy is linked to an orientation of the predominant axis (predominant direction of the diffusion). Post-processing programs are able to extract this directional information.
This additional information is difficult to represent on 2D grey-scaled images. To overcome this problem a color code is introduced . Basic colors can tell the observer how the fibers are oriented in a 3D-coordinate system: This is termed an "anisotropic map". The software could encode the colors in this way:
- Red indicates directions in the X axis: right to left or left to right.
- Green indicates directions in the Y axis: posterior to anterior or from anterior to posterior.
- Blue indicates directions in the Z axis: foot-to-head direction or vice versa
Notice that the technique is unable to discriminate the "positive" or "negative" direction in the same axis.
See also
References
- ↑ Filler, Aaron (2010). "The History, Development and Impact of Computed Imaging in Neurological Diagnosis and Neurosurgery: CT, MRI, and DTI". Internet Journal of Neurosurgery 7 (1).
- ↑ Basser, P. et al. (2000). "In Vivo Fiber Tractography Using DT-MRI Data.". Magnetic Resonance in Medicine 44: 625–632. doi:10.1002/1522-2594(200010)44:4<625::AID-MRM17>3.0.CO;2-O. PMID 11025519.
- ↑ Le Bihan D. and Breton,E. Imagerie de diffusion in vivo par résonance magnétique nucléaire. C.R.Acad.Sc.Paris T.301, Série II:1109-1112, 1985
- ↑ Le Bihan D., Breton E., Lallemand D., Grenier P., Cabanis E., Laval-Jeantet M. MR Imaging of Intravoxel Incoherent Motions: Application to Diffusion and Perfusion in Neurologic Disorders, Radiology, 161,401-407, 1986.
- ↑ Basser PJ, Mattiello J, Le Bihan D. MR Diffusion Tensor Spectroscopy and Imaging. Biophys. J. 66:259-267, 1994
- ↑ Basser PJ, Mattiello J, Le Bihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994 Mar;103(3):247-54.