Topterone
 | |
| Names | |
|---|---|
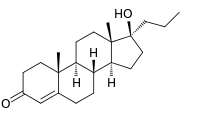
| IUPAC name
(8R,9S,10R,13S,14S,17S)-17-Hydroxy-10,13-dimethyl-17-propyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one | |
| Identifiers | |
| 60607-35-4 | |
| |
| Jmol-3D images | Image |
| PubChem | 9797605 |
| |
| Properties | |
| Molecular formula |
C22H34O2 |
| Molar mass | 330.50 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Topterone, WIN 17,665 (17 alpha-propyltestosterone) is an antiandrogen.[1]
Synthesis
Reaction of the tetrahydropyranyl ether of dehydroepiandrosterone with propyl magnesium bromide gives after removal of the protecting group the corresponding 17α-propyl derivative. Oppenauer oxidation leads to the corresponding conjugated ketone. There is thus obtained topterone.[2]
References
- ↑ Ferrari, RA; Chakrabarty, K; Creange, JE; Beyler, AL; Potts, OG; Schane, HP (1980). "Endocrine profile of topterone, a topical antiandrogen, in three species of laboratory animals". Methods and findings in experimental and clinical pharmacology 2 (2): 65–9. PMID 7339330.
- ↑ A. L. Beyler and R. A. Ferrari, DE 2633925 (1977).