Tolmetin
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
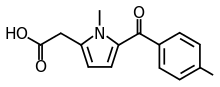
| [1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl]acetic acid | |
| Clinical data | |
| Trade names | Tolectin |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a681033 |
| |
| oral | |
| Pharmacokinetic data | |
| Half-life | 1-2 hours, next phase up to 5 hours |
| Identifiers | |
|
26171-23-3 | |
| M01AB03 M02AA21 | |
| PubChem | CID 5509 |
| DrugBank |
DB00500 |
| ChemSpider |
5308 |
| UNII |
D8K2JPN18B |
| KEGG |
D02355 |
| ChEBI |
CHEBI:71941 |
| ChEMBL |
CHEMBL1020 |
| Chemical data | |
| Formula | C15H15NO3 |
| 257.285 g/mol | |
|
SMILES
| |
| |
| | |
Tolmetin /ˈtɒlmətɨn/ is a non-steroidal anti-inflammatory drug of the arylalkanoic acids. It is used primarily to reduce hormones that cause pain, swelling, tenderness, and stiffness in conditions such as osteoarthritis and rheumatoid arthritis, including juvenile rheumatoid arthritis. In the United States it is marketed as Tolectin and comes as a tablet or capsule.
Clinical trials
Tolmetin is applicable in the treatment of rheumatoid arthritis,[1][2] osteoarthrosis,[3][4] pain,[5] and ankylosing spondylitis.[6]
Mechanism of action
Although the mechanism of action of tolmetin is unknown, research involving humans and animals has shown that tolmetin does not achieve anti-inflammatory response by stimulation of the adrenal or pituitary gland, but it has shown tolmetin restrains prostaglandin synthetase in vitro and reduces plasma levels of prostaglandin E, possibly causing the anti-inflammatory response.
When tested in rats tolmetin prevented experimentally stimulated polyarthritis and reduced inflammation. In patients with rheumatoid arthritis or osteoarthritis tolmetin restrained disease activity as efficiently as aspirin and indometacin, although the occurrence of mild gastrointestinal adverse effects and tinnitus was lower in patients treated with tolmetin than it was with aspirin-treated patients and the occurrence of adverse effects of the central nervous system was lower with tolmetin than it was with indometacin.[7]
Side effects
Tolmetin can increase the risk of heart or circulatory conditions such as heart attacks and strokes. It should not be taken shortly before or after coronary artery bypass surgery.[8] Tolmetin can also increase the risk of gastrointestinal conditions such as perforation or bleeding, which is fatal. Antacids can be taken with tolmetin to relieve stomachaches that often occur.[8] Overdose can result in drowsiness, nausea, epigastric pain, and vomiting.
Chemical synthesis
Tolmetin can be synthesized by the following route:[9]
- J. Carson, U.S. Pat. 3.752.826 (1973).
- J.R. Carson, Fr. Pat. 1.574.570 (1969)
References
- ↑ Cordrey L J (1976). "Tolmetin sodium, a new anti-arthritis drug: double-blind and long-term studies". Journal of the American Geriatrics Society 24 (10): 440–446. PMID 61224.
- ↑ Cardoe N; Steele C E (1976–1977). "A double-blind crossover comparison of tolmetin sodium and phenylbutazone in the treatment of rheumatoid arthritis". Current medical research and opinion 4 (10): 688–694. doi:10.1185/03007997609112003. PMID 800970.
- ↑ Liyanage S P; Steele C E (1977–1978). "Tolmetin in osteoarthrosis of the hip and knee: double-blind crossover trials". Current medical research and opinion 5 (4): 299–305. doi:10.1185/03007997709110184. PMID 343992.
- ↑ Davies, J; Dixon, AS; Steele, CE (1980). "Tolmetin sodium and indomethacin in the treatment of osteoarthrosis of the hip: a double-blind crossover study". Current medical research and opinion 7 (2): 115–20. doi:10.1185/03007998009112037. PMID 7002480.
- ↑ Stacher, G; Bauer, P; Ehn, I; Schreiber, E (1979). "Effects of tolmetin, paracetamol, and of two combinations of tolmetin and paracetamol as compared to placebo on experimentally induced pain. A double blind study". International journal of clinical pharmacology and biopharmacy 17 (6): 250–5. PMID 381221.
- ↑ Calin, A (1983). "Clinical use of tolmetin sodium in patients with ankylosing spondylitis: a review". Journal of clinical pharmacology 23 (7): 301–8. doi:10.1002/j.1552-4604.1983.tb02740.x. PMID 6350376.
- ↑ "Tolmetin". DrugBank. Retrieved 2007-07-02.
- ↑ 8.0 8.1 "Tolmetin". MedlinePlus. Archived from the original on 2007-06-10. Retrieved 2007-07-02.
- ↑ Carson, John R.; McKinstry, Doris N.; Wong, Stewart (1971). "5-Benzoyl-1-methylpyrrole-2-acetic acids as antiinflammatory agents". Journal of Medicinal Chemistry 14 (7): 646. doi:10.1021/jm00289a026.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||
