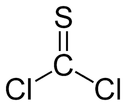
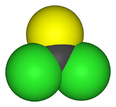
Thiophosgene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Carbonothioyl dichloride | |||
| Other names
Thiophosgene; Thiocarbonyl chloride; Carbonothioic dichloride | |||
| Identifiers | |||
| 463-71-8 | |||
| ChEBI | CHEBI:29366 | ||
| ChemSpider | 9645 | ||
| |||
| Jmol-3D images | Image | ||
| PubChem | 10040 | ||
| RTECS number | XN2450000 | ||
| |||
| UNII | 067FQP576P | ||
| Properties | |||
| CSCl2 | |||
| Molar mass | 114.98 g/mol | ||
| Appearance | Red liquid | ||
| Density | 1.50 g/cm3 | ||
| Boiling point | 70 to 75 °C (158 to 167 °F; 343 to 348 K) | ||
| Decomposition | |||
| Solubility in other solvents | polar organic solvents rxn with amines and alcohols | ||
| Refractive index (nD) |
1.558 | ||
| Structure | |||
| Molecular shape | planar, sp2, C2v | ||
| Hazards | |||
| Main hazards | Highly toxic | ||
| EU Index | Not listed | ||
| Flash point | 62 °C (144 °F; 335 K) | ||
| Related compounds | |||
| Related compounds |
Phosgene Thiocarbonyl fluoride Thiocarbonyl bromide Sulfur dichloride thionyl chloride | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Thiophosgene is a red liquid with the formula CSCl2. It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses.
Synthesis of CSCl2
CSCl2 is prepared in a two-step process from carbon disulfide. In the first step, carbon disulfide is chlorinated to give trichloromethanesulfenyl chloride, CCl3SCl:
- CS2 + 3 Cl2 → CCl3SCl + S2Cl2
The chlorination must be controlled as excess chlorine converts trichloromethanesulfenyl chloride into carbon tetrachloride. Steam distillation separates the trichloromethanesulfenyl chloride, a rare sulfenyl chloride, and hydrolyzes the sulfur monochloride. Reduction of trichloromethanesulfenyl chloride produces thiophosgene:
- CCl3SCl + M → CSCl2 + MCl2
Typically, tin is used for the reducing agent M.[1]
Uses of CSCl2
CSCl2 is mainly used to prepare compounds with the connectivity CSX2 where X = OR, NHR. Such reactions proceed via intermediate such as CSClX. Under certain conditions, one can convert primary amines into isothiocyanates. CSCl2 also serves as a dienophile to give, after reduction 5-thiacyclohexene derivatives. Thiophosgene is also known as the appropriate reagent in Corey-Winter synthesis for stereospecific conversion of 1,2-diols into olefins.[2]
Safety considerations
CSCl2 is considered highly toxic.
References
- ↑ Dyson, G. M. (1926). "Thiophosgene" (PDF). Org. Synth. 6: 86. doi:10.15227/orgsyn.006.0086.; Coll. Vol. 1, p. 506
- ↑ Sharma, S. (1978). "Thiophosgene in Organic Synthesis". Synthesis 1978 (11): 803–820. doi:10.1055/s-1978-24896.
- Holleman, A. F.; Wiberg, E. (2001), Inorganic Chemistry, San Diego: Academic Press, ISBN 0-12-352651-5